1. INTRODUCTION
Erythrodermic psoriasis is characterized by diffuse erythema and scaling that can affect total body surface area and cause sepsis and death without treatment. 1 Vaccination has been associated with both guttate and plaque psoriasis flare‐ups. 2 To date, we have not found a documented case of erythrodermic psoriasis flare due to the SARS‐CoV‐2 vaccine. Here, we report the observation of an erythrodermic psoriasis eruption in an adult patient who received the first dose of the Pfizer‐BioNTech SARS‐CoV‐2 vaccine.
2. REPORT OF A CASE
A 58‐year‐old male presented to the hospital for 6 days of generalized rash covering his body with partial sparing of the medial thighs and feet. It started a day after he received the Pfizer‐BioNTech SARS‐CoV‐2 vaccine. Past medical history was pertinent for guttate psoriasis with occasional flares but mainly controlled with topical corticosteroids. He denied using any new medications or changes to drugs, changes in diet, or contact with a possible allergen or irritant. Other than the presenting symptoms, the review of systems was negative.
At presentation, vital signs were normal. There was confluent bright erythema on the exam with a thick and loose yellow‐gray scale on the patient's face, torso, and upper extremities. Annular erythematous plaques coalesced into confluent plaques with hyperkeratotic scales on the lower extremities and buttocks. (Figure 1) The remainder of the mucocutaneous, lymphatic, abdominal, and cardiopulmonary exams were unremarkable. Laboratory testing demonstrated a mild leukocytosis (11.48x103/μL; reference: 4.20–10.70 × 103/μL), with mature granulocytosis (8.35 × 103/μL; reference: 1.99–6.95 × 103/μL) and mild thrombocytosis (405 × 103/μL; reference: 150‐328 × 103/μL). Creatinine was elevated and consistent with an acute kidney injury (1.66 mg/dL; reference: 0.6–1.25 mg/dL). Liver function tests and electrolytes were within normal limits. Microbiological results revealed negative SARS‐CoV‐2 nasopharyngeal PCR, negative blood cultures, immunity to hepatitis B, and negative serologies for hepatitis A/C, HIV, and syphilis.
FIGURE 1.
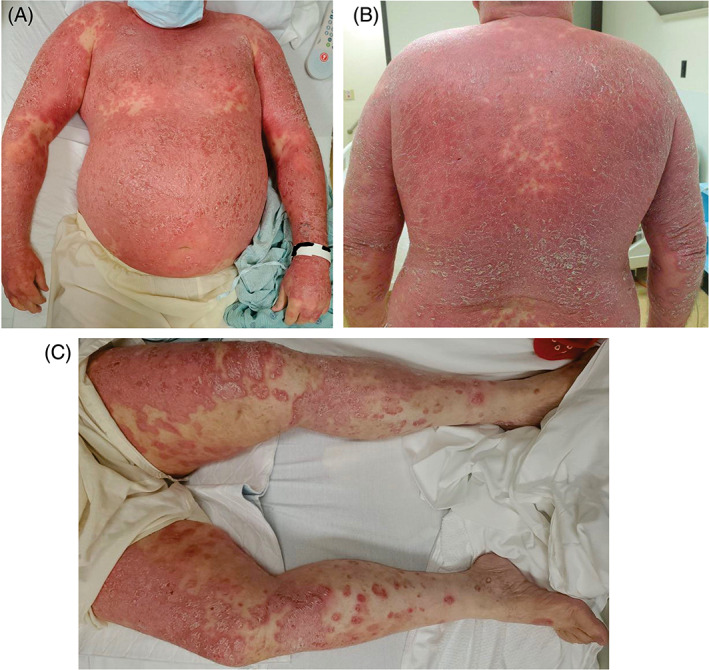
Initial presentation 6 days after onset of rash and 7 days after the initial dose of SARS‐CoV‐2 vaccine. (A) Confluent plaques on the torso and arms. (B) Confluent plaques on the back. (C) Annular erythematous plaques with some coalescing into confluent plaques with hyperkeratotic scales on the lower extremities with partial sparing of the medial thighs and sparing of the feet
A punch biopsy of the left arm showed psoriasiform dermatitis. Therefore, a diagnosis of erythrodermic psoriasis secondary to immunization was made. Following the resolution of his AKI, he was started on cyclosporine 3 mg/kg/day with UVB treatments 3 times a week for 3 months and twice‐daily triamcinolone 0.1% cream and hydroxyzine as needed. A week following treatment initiation, drastic improvement was noted with significant sloughing of skin that revealed new, non‐irritated skin underneath, and the 3‐month follow‐up revealed complete resolution.
3. DISCUSSION
Most reported psoriasis flares associated with vaccination have been classified as guttate and plaque variants. 2 , 3 This case describes an erythrodermic psoriasis eruption caused by the SARS‐CoV‐2 vaccination. mRNA vaccines stimulate innate and adaptive immune responses by producing SARS‐CoV‐2 spike (S) protein. 4 Production of S protein primes T cells (both CD8+ and CD4+) to differentiate and generate cytotoxic and inflammatory mediators and antibody‐secreting plasma cells. Specifically, the previously reported detection of IFN‐ɣ, IL‐2, and IL‐12p70 indicates a dominant Th1 profile. 5 Cytokines produced by Th1 cells are potent activators of keratinocytes. The amount of IFN‐ɣ has been positively correlated with the psoriasis severity index. 6 Therefore, the Th1 enhanced response could present a possible mechanism for the observed flare‐up.
Our case highlights a rare complication of the SARS‐CoV‐2 vaccine. The extremely low incidence of this complication should not affect the immunization guidelines for patients with psoriasis, especially those immunocompromised due to immunosuppressive therapy. However, it is imperative to assess each patient's risk and benefit, as the SARS‐CoV‐2 vaccine can be a triggering factor for erythrodermic psoriasis, which can have devastating clinical sequelae.
INFORMED CONSENT
We obtained informed consent from the patient.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
FUNDING INFORMATION
None.
AUTHOR CONTRIBUTIONS
All authors had access to the data and contributed to writing this manuscript.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Lebwohl M. Psoriasis. Lancet. 2003;361(9364):1197‐1204. doi: 10.1016/S0140-6736(03)12954-6 [DOI] [PubMed] [Google Scholar]
- 2. Sotiriou E, Tsentemeidou A, Bakirtzi K, Lallas A, Ioannides D, Vakirlis E. Psoriasis exacerbation after COVID‐19 vaccination: a report of 14 cases from a single centre. J Eur Acad Dermatol Venereol. 2021;35(12):e857‐e859. doi: 10.1111/jdv.17582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gunes AT, Fetil E, Akarsu S, Ozbagcivan O, Babayeva L. Possible triggering effect of influenza vaccination on psoriasis. J Immunol Res. 2015;2015:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2021;384(5):403‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sahin U, Muik A, Derhovanessian E, et al. COVID‐19 vaccine BNT162b1 elicits human antibody and TH 1 T cell responses. Nature. 2020;586(7830):594‐599. [DOI] [PubMed] [Google Scholar]
- 6. Kurtovic NO, Halilovic EK. Serum concentrations of interferon gamma (IFN‐γ) in patients with psoriasis: correlation with clinical type and severity of the disease. Mediev Archaeol. 2018;72(6):410‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


