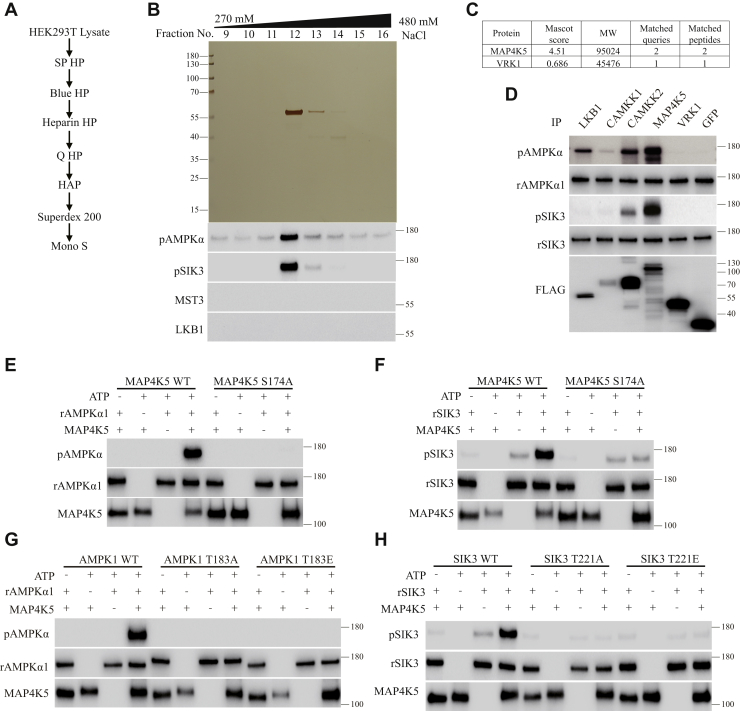
Figure 3.
Purification of MAP4K5 as an AMPKα1-T183 and SIK3-T221 phosphorylating activity from HEK cells.A, a diagram for our third round of purification. The focus was on fraction 12 (330 mM NaCl) from the first (SP HP) column (Fig. S2). B, silver staining and phosphorylation assays of fractions from the Mono S column. Fractions were dialyzed against buffer A and followed by silver staining and in vitro phosphorylation of AMPKα1-T183 and SIK3-T221. LKB1 and MST3 were examined but not detected in these fractions. The single visible bands in fractions 12 and 13 (330–360 mM NaCl) were isolated for MS analysis. C, two protein serine/threonine kinases (MAP4K5 and VRK1) were found by MS analysis. D, LKB1, CaMKK1, CaMKK2, MAP4K5, VRK1, and GFP were individually expressed as FLAG-tagged proteins in HEK cells and immunoprecipitated. They were tested for phosphorylation of recombinant AMPKα1 at T183 and recombinant SIK3 at T221. MAP4K5 phosphorylated both AMPKα1-T183 and SIK3-T221. LKB1 and CaMKK2 phosphorylated AMPKα1-T183 more strongly than SIK3-T221. VRK1 and GFP phosphorylated neither AMPKα1-T183 nor SIK3-T221. E, recombinant AMPKα1 was phosphorylated at T183 by MAP4K5 WT but not its S174A mutant immunoprecipitated from HEK cells. F, recombinant SIK3 was phosphorylated at T221 by MAP4K5 WT but not its S174A mutant immunoprecipitated from HEK cells. G, recombinant AMPKα1 WT, but not AMPKα1 T183A or AMPKα1 T183E, was phosphorylated by MAP4K5 WT immunoprecipitated from HEK cells. H, recombinant SIK3 T221, but not SIK3 T221A or SIK3 T221E, was phosphorylated by MAP4K5 WT immunoprecipitated from HEK cells. AMPK, AMP-activated protein kinase; CaMKK2, Ca2+/calmodulin-dependent protein kinase kinase 2; HEK, human embryonic kidney cell; LKB1, liver kinase B1; MAP4K5, mitogen-activated protein kinase kinase kinase kinase 5; MS, mass spectrometry; SIK3, salt-inducible kinase 3.