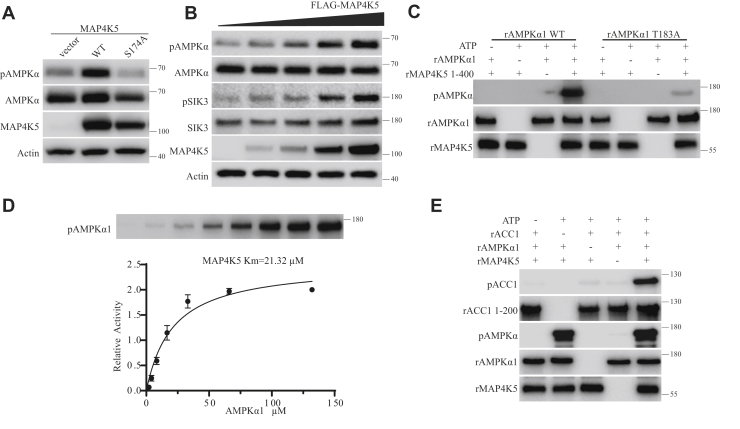
Figure 4.
AMPKα and SIK3 phosphorylation by MAP4K5 in vivo and in vitro.A, control vector or plasmids encoding MAP4K5 WT or its S174A mutant were transfected into Lkb1 and Camkk2 double KO HEK293T cells. MAP4K5 WT but not its S174A mutant increased phosphorylation of endogenous AMPKα T172 in HEK cells. B, increasing concentrations of the plasmid expressing a FLAG-tagged MAP4K5 were transfected into Lkb1 and Camkk2 double KO HEK cells. After 24 h, cells were starved with DMEM without glucose and glutamine for 1 h before lysis and Western analysis. Increasing amounts of the plasmid led to increasing expression of FLAG-MAP4K5 protein and correspondingly increased phosphorylation of endogenous AMPKα-T172 and SIK3-T221 in HEK cells, in a manner independent of both LKB1 and CaMKK2. C, recombinant MAP4K5 expressed in Escherichia coli could directly phosphorylate recombinant AMPKα1 WT at T183 but not recombinant AMPKα1 T183A mutant. D, the Michaelis–Menten constant of recombinant MAP4K5 on recombinant AMPKα1 was 21.32 μM. E, recombinant MAP4K5 increased the catalytic activity of recombinant AMPKα1 on its substrate recombinant ACC1. The procedure was similar to that described in Figure 5J in the companion article. Prior treatment of recombinant AMPKα1 by recombinant MAP4K5 increased AMPKα1 catalyzed phosphorylation of recombinant ACC1. AMPK, AMP-activated protein kinase; CaMKK2, Ca2+/calmodulin-dependent protein kinase kinase 2; DMEM, Dulbecco's modified Eagle's medium; HEK293T, human embryonic kidney 293T cell; LKB1, liver kinase B1; MAP4K5, mitogen-activated protein kinase kinase kinase kinase 5; SIK3, salt-inducible kinase 3.