Key Points
Question
Were the outcomes associated with a physician champion-led, electronic health record–embedded clinical decision support intervention for risk-stratifying adults presenting at the emergency department (ED) with acute pulmonary embolism sustained 4 years after initial promotion?
Findings
In this cohort study of 1039 patients across 21 EDs, outpatient management increased significantly compared with prior practices in former control EDs. Former intervention sites continued to outperform former controls in managing acute pulmonary embolism among patients with low risk.
Meaning
These findings suggest that this champion-led, clinical decision support intervention was associated with sustained practice change in identifying and safely discharging patients with low-risk pulmonary embolism.
This cohort study evaluates the sustainability of recommended site-of-care decision-making support 4 years after an initial intervention to increase outpatient management of acute pulmonary embolism among patients presenting to the emergency department (ED).
Abstract
Importance
Physicians commonly hospitalize patients presenting to the emergency department (ED) with acute pulmonary embolism (PE), despite eligibility for safe outpatient management. Risk stratification using electronic health record–embedded clinical decision support systems can aid physician site-of-care decision-making and increase safe outpatient management. The long-term sustainability of early improvements after the cessation of trial-based, champion-led promotion is uncertain.
Objective
To evaluate the sustainability of recommended site-of-care decision-making support 4 years after initial physician champion-led interventions to increase outpatient management for patients with acute PE.
Design, Setting, and Participants
This retrospective cohort study was conducted in 21 US community hospitals in an integrated health system. Participants included adult patients presenting to the ED with acute PE. Study sites had participated in an original decision-support intervention trial 4 years prior to the current study period: 10 sites were intervention sites, 11 sites were controls. In that trial, decision support with champion promotion resulted in significantly higher outpatient management at intervention sites compared with controls. After trial completion, all study sites were given continued access to a modified decision-support tool without further champion-led outreach. Data were analyzed from January 2019 to February 2020.
Exposures
ED treatment with a modified clinical decision support tool.
Main Outcomes and Measures
The main outcome was frequency of outpatient management, defined as discharge home directly from the ED, stratified by the PE Severity Index. The safety measure of outpatient care was 7-day PE-related hospitalization.
Results
This study included 1039 patients, including 533 (51.3%) women, with a median (IQR) age of 65 (52-74) years. Nearly half (474 patients [45.6%]) were rated lower risk on the PE Severity Index. Overall, 278 patients (26.8%) were treated as outpatients, with only four 7-day PE-related hospitalizations (1.4%; 95% CI, 0.4%-3.6%). The practice gap in outpatient management created by the earlier trial persisted in the outpatient management for patients with lower risk: 109 of 236 patients (46.2%) at former intervention sites vs 81 of 238 patients (34.0%) at former control sites (difference, 12.2; [95% CI, 3.4-20.9] percentage points; P = .007), with wide interfacility variation (range, 7.1%-47.1%).
Conclusions and Relevance
In this cohort study, a champion-led, decision-support intervention to increase outpatient management for patients presenting to the ED with acute pulmonary embolism was associated with sustained higher rates of outpatient management 4 years later. The application of our findings to improving sustainability of practice change for other clinical conditions warrants further study.
Introduction
Patients with low-risk pulmonary embolism (PE) can be safely treated without hospitalization.1,2,3,4,5,6,7,8 Outpatient management better stewards health care resources and helps patients avoid the cost, inconvenience, and risk associated with hospitalization.7,9,10 Society guidelines recommend outpatient management for patients with low risk.11,12,13,14 However, US physicians have been slow to embrace these recommendations.15 Operational barriers include difficult access to pharmacotherapy and timely follow-up. Physician barriers include discomfort with the unfamiliar, aversion to complexity, and concern about medicolegal risks.16
In 2014, we implemented the electronic Support for Pulmonary Embolism Emergency Disposition (eSPEED) trial to help overcome physician barriers in a health care system with ready access to pharmacotherapy and timely follow-up.7 Intervention emergency departments (EDs) had a web-based clinical decision-support system (CDSS) integrated into the electronic health record (EHR) to provide evidence-based, risk-stratified recommendations to guide site-of-care decision-making for emergency physicians treating ED patients with acute PE. Onsite peer champions provided physician education, CDSS promotion, audit and feedback, and role modeling. During an 8-month intervention period, outpatient management safely increased in intervention EDs without any change in controls.7
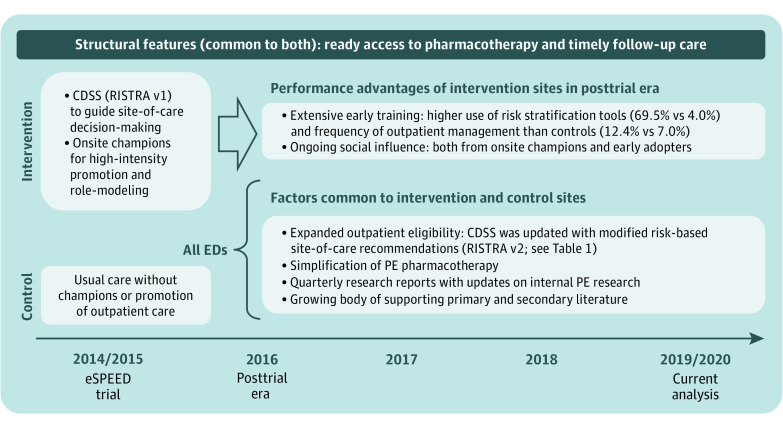
EHR-embedded decision support can improve physician decision-making,17,18 even in busy EDs.17,19 But effect durability has not been well studied. To be sustainable, practice innovations must continue their impact in the absence of their initial promotion and must adapt to changing conditions and evidence.20,21 With this in mind, we updated CDSS site-of-care recommendations in 2016 to reflect our trial results, broadening outpatient eligibility without changing hospitalization indications. We gave decision-support access to control EDs with a 1-hour educational and training session. However, after eSPEED, no EDs received structured promotion, and control EDs were never assigned a champion (Figure 1).
Figure 1. Timeline of Factors Facilitating Outpatient Management of Emergency Department Patients with Acute Pulmonary Embolism (PE) in Intervention and Control Sites.
The electronic Support for Pulmonary Embolism Emergency Disposition (eSPEED) trial assigned 10 emergency departments (EDs) to the intervention group and 11 to the control group based on the presence of an onsite study champion. The intervention included use of a web-based clinical decision support system (CDSS) for risk stratification called RISTRA integrated into the ED navigator of the electronic health record. RISTRA version 1 (v1) was introduced at intervention sites in late 2014 and v2 was introduced to intervention sites in 2016 and control sites in 2017.
In this 14-month retrospective cohort study in 2019 to 2020 across 21 EDs, we compared frequencies of outpatient management between former intervention and control EDs. We hypothesized that performance gains experienced earlier by intervention EDs would be sustained and that posttrial interventions would be associated with increased outpatient management in both ED groups. Lessons from this study may assist clinicians in providing the level of care that matches patient risk for those with acute PE.
Methods
This cohort study was approved by the Kaiser Permanente Northern California (KPNC) institutional review board with a waiver for the requirement for written informed consent because of the observational nature of the study in the course of usual care. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Design and Setting
This Sustained Effects (SUS-EFX) Study was a retrospective cohort study from January 2019 to February 2020 of all 21 community-based EDs of KPNC, an integrated health system that serves more than 4.5 million members who represent the surrounding racial, ethnic, and socioeconomic diversity of California.22 KPNC uses a comprehensive EHR, including outpatient, emergency, inpatient, laboratory, imaging, and pharmacy history.
The included 21 EDs are staffed by board-certified (or board-eligible) emergency physicians. In KPNC, PE is usually diagnosed in the ED; patients diagnosed in the clinic setting are commonly referred to an ED for definitive care.1 The system recommended direct oral anticoagulants (DOACs) for the treatment of most patients with acute PE (eAppendix 1 in the Supplement). Patients received timely follow-up23 and had access to anticoagulants with long-term monitoring by a pharmacy-led telephone-based anticoagulation management service,24,25,26 which contacts patients for education shortly after ED discharge. More information on ready access to pharmacotherapy and close follow-up is provided in eAppendix 2 in the Supplement.
We used evidence-based principles to design and build a web-based, EHR-integrated CDSS that we call RISTRA-PE (for risk stratification).27,28,29,30 It is accessible within the ED navigator of the EHR. Activation of RISTRA-PE after diagnostic confirmation of PE is physician-driven and entirely voluntary. There is no prompt or best-practice alert. RISTRA-PE includes an autopopulating version of the validated PE Severity Index (eTable 1 in the Supplement)8,31,32,55 with risk-based recommendations to inform site-of-care decision-making (Table 1) and outpatient exclusion criteria modeled after the Canadian criteria and Hestia clinical decision rule (eFigure in the Supplement).34,35 Our site-of-care recommendations were designed to be assistive, not directive (eAppendix 3 in the Supplement).36 The presence of right ventricular dysfunction was among our exclusion criteria, given the associated increase in short-term risk, even among patients with low risk.37,38,39,40 As with the 2016 CHEST guidelines,41 we did not recommend routine assessment of right ventricular dysfunction.
Table 1. Changing Risk-Based Site-of-Care Recommendations for Emergency Department Patients With Acute Pulmonary Embolism.
| Pulmonary Embolism Severity Index Score, points (Class)d | RISTRA-PE version 1a | RISTRA-PE version 2b | |||
|---|---|---|---|---|---|
| Approximate 30-d all-cause mortality, %e | Initial care recommendation | All-cause mortality, %c | Initial care recommendation | ||
| 7-d | 30-d | ||||
| ≤64 (I) | <2 | Outpatient management is often possible | 0 | 0 | Outpatient management is often appropriate |
| 65-85 (II) | <2 | Outpatient management is often possible | <1 | <1 | Outpatient management is often appropriate |
| 86-105 (III) | 5 | Inpatient care is often indicated | <1 | 3 | Outpatient management may be possible |
| 106-125 (IV) | 10 | Inpatient care is often indicated | <1 | 5 | Outpatient management may be possible |
| ≥126 (V) | 20 | Inpatient care is often indicated | 5 | 13 | Inpatient care is often indicated |
Abbreviations: ED, emergency department; RISTRA-PE, Risk Stratification for Pulmonary Embolism.
Launched September 2014 and promoted at 10 intervention EDs.
Accessible March 2017 to all 21 EDs.
Estimates based on internal data from the electronic Support for Pulmonary Embolism Emergency Disposition7 trial and associated studies.56
The Pulmonary Embolism Severity Index is presented in eTable 1 in the Supplement.
Estimates based on the Pulmonary Embolism Severity Index literature as of 2014.7
The 8-month eSPEED intervention ran from September 2014 through April 2015.7 Site assignment was not randomized. The 10 EDs that already had an onsite clinical champion received RISTRA-PE access (the original version 1) with champion-led promotion. These constituted the intervention sites. The other 11 EDs did not have an onsite champion and served as concurrent controls. Champions provided iterative physician education, personalized audit and feedback (eAppendix 4 in the Supplement), monthly emails reporting facility enrollment rates that commended leading and new enrollers, and small incentives for each physician’s first 3 enrollments. Champions also served as ED role models.7,42,43 Although CDSS use was unprompted, uptake across intervention sites was high (68.9%).44 Outpatient PE management, broadly defined as discharge home from the ED or an outpatient observation unit within 24 hours,45 increased at 10 intervention EDs, from 17.8% of patients receiving outpatient PE management to 28.3% of patients receiving outpatient PE management (a relative 59% increase). Restricting analysis to only ED discharges, the frequency increased from 7.8% of patients discharged home to 12.4% of patients discharged home. There was no increase in discharge home at 11 control EDs: 8.0% of patients were discharged home during the preintervention period, and 7.0% of patients were discharged home in the postintervention period.7
Posttrial Interventions
After completion of the eSPEED trial, we first updated RISTRA-PE’s risk-specific, site-of-care recommendations using trial results (starting in April 2016), expanding the scope of outpatient eligibility without changing outpatient exclusion criteria (Table 1; eFigure in the Supplement). Cycling internal study results back into practice-change interventions is a learning health system goal and a requirement for sustainability.46,47 We subsequently provided access to RISTRA-PE version 2 across all 21 EDs, including controls, starting in February 2017 (Figure 1), introducing the tool with an emailed set of educational slides followed by an hour-long in-person educational presentation, part of a required 1-day educational forum with nearly 100% attendance. Champions continued to provide patient care at intervention EDs and may have continued to exert social influence (along with early adopters) among their immediate peers.48,49,50,51 However, champions provided no structured promotion of RISTRA-PE version 2: no further emails, departmental presentations, enrollment incentives, or audit and feedback were provided. There was minimal crossover of emergency physicians (<2%) working an occasional shift outside their home EDs, eg, intervention physicians at control sites.
Second, we disseminated quarterly research reports to the ED physicians with updates of our ongoing PE studies (Figure 1).7,23,32,33,36,52,53,54,55,56 Continued research from our own practice setting on the safety and effectiveness of outpatient management for patients with acute PE may have helped communicate that this was becoming a systemwide standard of care.57
Third, the medical group switched pharmacotherapy recommendations from warfarin to DOACs in 2016 in concert with CHEST guidelines.41 While DOACs alone may be insufficient in shifting ED site-of-care practices,58 simplifying pharmacotherapy might have a supportive association in systems already primed for outpatient care.
Study Population
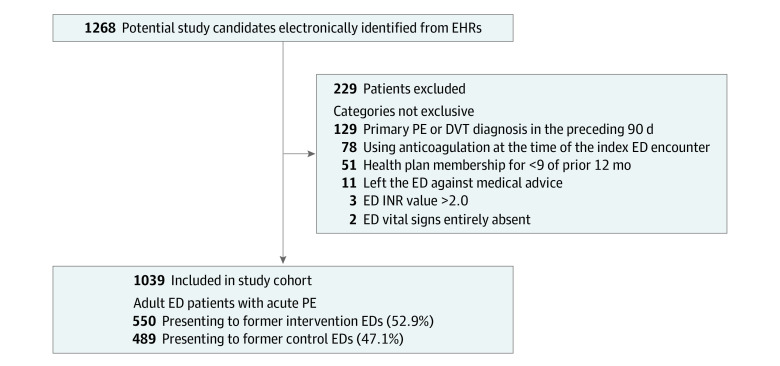
In the SUS-EFX study, we included health plan members aged 18 years or older with a primary diagnosis of PE in the ED accompanied by positive results for PE in computed tomography (CT) or scintigraphy imaging (either in the ED or within the prior 12 hours) from January 2019 through February 2020. We used validated natural language processing algorithms to identify positive results in CT pulmonary angiography and ventilation/perfusion scintigraphy.1 Patients with any of the following were excluded: a diagnosis of acute venous thromboembolism in the previous 90 days, using anticoagulants at the time of diagnosis (or an elevated ED international normalized ratio >2.0), lack of adequate health plan membership in the prior 12 months (as this affects completeness of medical history), leaving the ED against medical advice, absence of any documented ED vital signs (precluding calculation of the PE Severity Index), or known pregnancy.
Data Collection and Study Outcomes
We obtained study demographic and clinical variables directly from the health system’s EHR using automated electronic data extraction. Race and ethnicity were self-reported and included to demonstrate that the diversity of the cohort reflects the population of northern California. We used the validated 11-variable PE Severity Index (eTable 1 in the Supplement) to estimate 30-day all-cause mortality, as previously described, and to stratify our primary outcome.7,32,54,56 Altered mental status was the only PE Severity Index variable not reliably available. For our analysis, we assumed results were negative (eAppendix 5 in the Supplement), as other studies of the PE Severity Index have done, including the original validation studies.59,60 We performed manual EHR review of approximately 10% of patients (109 patients), some randomly selected and others from targeted subpopulations, to validate 2 study variables (ie, study eligibility and initial site of care) and to adjudicate the primary safety outcome (7-day PE-related hospitalization) among outpatients, along with their 30-day all-cause mortality (eAppendix 6 in the Supplement).
Our primary study outcome was outpatient management, defined as discharge to home directly from the ED. We compared our results by original eSPEED trial assignment (intervention vs control) to evaluate the association of earlier trial assignment with recent site-of-care practices, stratified by risk classification based on the PE Severity Index. Our primary safety outcome for outpatients was 7-day hospitalization for PE-related signs, symptoms, or interventions, defined a priori and used in earlier studies (eAppendix 7 in the Supplement).7,56 We used claims data to identify hospitalizations outside the health system. We also measured mortality using the health system mortality database that links to the Social Security death master file and the California State Department of Vital Statistics. State mortality reports from 2020 were not yet available.
Statistical Analysis
We used Wilcoxon nonparametric tests for continuous variables and χ2 tests for categorical variables to compare patient characteristics between intervention and control EDs. We reported the frequency of outpatient management and compared the difference between intervention and control EDs overall. To account for potential confounders, we compared the results between intervention and control sites stratified by the PE Severity Index, as it guided our site-of-care recommendations (Table 1) and is associated with 30-day all-cause mortality.54 We planned to adjust for other covariates (beyond those of the PE Severity Index) if any were found to be significantly different on bivariate analysis. We considered a 2-tailed P < .05 to be significant. We reported the incidence of 7-day PE-related hospitalization among those managed as outpatients and 30-day mortality among all patients. Since we did not have complete state-reported mortality data for patients diagnosed during the last 3 months of the study period (December 2019 to February 2020), we performed a sensitivity analysis of mortality outcomes by excluding these months. The number of patients during the study period determined the sample size. All analyses were conducted with SAS statistical software version 9.4 (SAS Institute). Data were analyzed from January 2019 to February 2020.
Results
We identified 1268 adults presenting to the ED with a PE diagnosis and positive results in a pulmonary imaging study. We excluded 229 patients, most commonly because of recent thromboembolic disease, anticoagulation, and insufficient prior health plan membership (Figure 2). The remaining 1039 patients had 1032 study-eligible encounters (7 patients had 2 eligible encounters each). In total, 553 patients (51.3%) were women, and the median (IQR) age was 65 (52-74) years (Table 2). A total of 150 patients (14.4%) were African American, 65 patients (6.3%) were Asian, 110 patients (10.6%) were Hispanic or Latinx, and 707 patients (68.1%) were White. Most patients were diagnosed using CT pulmonary angiogram (1025 patients [98.7%]). Nearly half of all patients were lower risk on the PE Severity Index (classes I-II; 474 patients [45.6%]). Overall, 278 patients (26.8%) received outpatient PE management after a median (IQR) ED length of stay of 4.7 (3.6-6.0) hours. Also, 367 patients (35.3%) were discharged home from either the ED or an outpatient observation unit, and 401 patients (38.6%) were discharged home within 24 hours of ED registration.45 Patients selected for outpatient care were younger, less commonly arrived by ambulance, had markedly anomalous vital signs or elevated troponin concentrations, and more commonly arrived with pre-ED imaging or were in lower-risk classes than their hospitalized counterparts (eTable 2 in the Supplement).7,56,61 The median (IQR) ED frequency of outpatient management was 29.0% (21.5%-31.8%), and the frequency varied widely between EDs (range, 7.1%-47.1%).
Figure 2. Cohort Assembly and Initial Site of Care for Adult Emergency Department (ED) Patients With Acute Pulmonary Embolism (PE).
DVT indicates deep vein thrombosis; and INR, international normalized ratio.
Table 2. ED Patients With Acute Pulmonary Embolism Stratified by Prior Trial Assignment in 2014.
| Characteristics | Patients, No. (%) | P value | ||
|---|---|---|---|---|
| Total cohort (N = 1039) | eSPEED trial assignment a | |||
| Intervention (n = 550) | Control (n = 489) | |||
| Age, median (IQR) | 65 (52-74) | 66 (52-75) | 64 (52-74) | .08 |
| Sex | ||||
| Women | 533 (51.3) | 287 (52.2) | 246 (50.3) | .55 |
| Men | 506 (48.7) | 263 (47.8) | 243 (49.7) | |
| Race and ethnicityb | ||||
| African American | 150 (14.4) | 85 (15.5) | 65 (13.3) | .48 |
| Asian | 65 (6.3) | 36 (6.6) | 29 (5.9) | |
| Hispanic or Latinx | 110 (10.6) | 50 (9.1) | 60 (12.3) | |
| White | 707 (68.1) | 375 (68.2) | 332 (67.9) | |
| Other | 7 (0.7) | 4 (0.7) | 3 (0.6) | |
| Comorbidities | ||||
| Chronic lung disease | 275 (26.5) | 145 (26.4) | 130 (26.6) | .94 |
| Cancer (active or history) | 252 (24.3) | 150 (27.3) | 102 (20.9) | .02 |
| Heart failure (systolic or diastolic) | 43 (4.1) | 26 (4.7) | 17 (3.5) | .31 |
| Arrival by ambulance | 188 (18.1) | 102 (18.6) | 86 (17.6) | .69 |
| Worst vital signsc | ||||
| Systolic blood pressure <100 mm Hg | 162 (15.6) | 104 (18.9) | 58 (11.9) | .002 |
| Heart rate ≥110 beats/min | 278 (26.8) | 165 (30.0) | 113 (23.1) | .01 |
| Respiratory rate ≥30 breaths/min | 120 (11.6) | 73 (13.3) | 47 (9.6) | .07 |
| Pulse oximetry <90%d | 140 (13.5) | 75 (13.6) | 65 (13.3) | .87 |
| Temperature <36 °C | 19 (1.8) | 10 (1.8) | 9 (1.8) | .98 |
| Diagnostic imaging, timing | ||||
| Prearrival (<12h) | 107 (10.3) | 55 (10.0) | 52 (10.6) | .74 |
| ED | 932 (89.7) | 495 (90.0) | 437 (89.4) | |
| PE Severity Index classificatione | ||||
| I-II (lower risk) | 474 (45.6) | 236 (42.9) | 238 (48.7) | <.001 |
| III-IV (intermediate risk) | 393 (37.8) | 199 (36.2) | 194 (39.7) | |
| V (highest risk) | 172 (16.6) | 115 (20.9) | 57 (11.7) | |
| Troponin I concentrationf | ||||
| Within reference range | 630 (60.6) | 324 (58.9) | 306 (62.6) | .10 |
| Elevated | 263 (25.3) | 154 (28.0) | 109 (22.3) | |
| Not performed | 146 (14.1) | 72 (13.1) | 74 (15.1) | |
Abbreviations: ED, emergency department; eSPEED, electronic Support for Pulmonary Embolism Emergency Disposition; PE, pulmonary embolism.7
EDs were assigned to the intervention (10 EDs) or control (11 EDs) groups based on the presence of an onsite study champion.
Race and ethnicity were self-reported. Other race and ethnicity includes Native American and Hawaiian and Pacific Islander patients.
Worst in the direction in question measured during the ED encounter. Missing values were uncommon: 0 patients were missing systolic blood pressure; 1 patient (0.1%) was missing pulse rate; 1 patient (0.1%) was missing respiratory rate; 2 patients (0.2%) were missing pulse oximetry; and 26 patients (2.5%) were missing temperature. These percentages are similar to those in the eSPEED trial. Missing vital signs were comparable between intervention and control sites.
With or without oxygen supplementation.
More information on the PE Severity Index is presented in eTable 1 in the Supplement.
Highest concentration during the ED encounter.
Patients with acute PE managed in EDs that had been former eSPEED intervention sites had a higher prevalence of cancer, anomalous vital signs, and higher mortality risk scores compared with controls. No other covariates were significantly different (Table 2). These findings supported our a priori strategy to stratify comparisons by risk classification. The 2 ED groups were comparable in terms of census, hospitalization rates, hospital bed capacity, and access to observation units, as previously reported.7
Overall, the frequency of outpatient management was similar between intervention and control sites (although intervention sites had patients with higher risk): 156 patients (28.4%) at intervention sites vs 122 patients (25.0%) at control sites (difference, 3.4 [95% CI, −2.2 to 8.8] percentage points; P = .21) (Table 3). These frequencies were higher than when measured during the eSPEED trial 4 years earlier: 12.4% in the intervention sites vs 7.0% in the control sites. When evaluated by risk strata, the intervention EDs outperformed their control counterparts among patients with lower risk: 109 patients (46.2%) vs 81 patients (34.0%) (difference, 12.2 [95% CI, 3.4 to 20.9] percentage points; P = .007), with no statistically significant differences among patients with higher risk (Table 3). Patients with lower risk were most likely to reflect changes in site-of-care practices because they were more commonly eligible for outpatient management and were strongly recommended for outpatient care by RISTRA-PE version 2.
Table 3. Frequency of Outpatient Management of Emergency Department Patients With Acute Pulmonary Embolism Stratified by 30-Day All-Cause Mortality Risk Classification.
| Risk group | Patients receiving outpatient management, No./total No. (%)a | Difference, percentage points (95% CI) | ||
|---|---|---|---|---|
| Total cohort (N = 1039) | ED assignment during eSPEED Trial | |||
| Intervention (n = 550) | Control (n = 489) | |||
| All, No. (%) | 278 (26.8) | 156 (28.4) | 122 (24.9) | 3.4 (−2.0 to 8.8) |
| By risk stratab | ||||
| Lower risk | 190/474 (40.1) | 109/236 (46.2) | 81/238 (34.0) | 12.2 (3.4 to 20.9) |
| Intermediate risk | 74/393 (18.8) | 36/199 (18.1) | 38/194 (19.6) | −1.5 (−9.2 to 6.2) |
| Highest risk | 14/172 (8.1) | 11/115 (9.6) | 3/57 (5.3) | 4.3 (−3.6 to 12.2) |
Abbreviations: ED, emergency department; eSPEED, electronic Support for Pulmonary Embolism Emergency Disposition.7
Outpatient management was defined as discharge home directly from the ED. Observation unit admission was categorized as hospitalization.
Thirty-day all-cause mortality risk was estimated from validated Pulmonary Embolism Severity Index classification, with lower risk including classes I and II; intermediate risk, classes III and IV; and highest risk, class V.
The incidence of 7-day PE-related hospitalization among outpatients was low (4 patients [1.4%; 95% CI, 0.4 to 3.6]): 3 patients were treated in an intervention ED and 1 patient was treated in a control ED (eTable 3 in the Supplement). Overall, 30-day all-cause mortality was 4.3%, similar to prior studies,7,56 and varied by site of care and risk class (eAppendix 8 and eTable 4 in the Supplement).
Physician use of RISTRA-PE version 2 for eligible ED patients was different between ED groups during the SUS-EFX study period. The tool was used for 62 of 550 physicians (11.3%) at intervention sites vs 36 of 489 physicians (7.4%) at control sites (P = .03). RISTRA-PE use during the SUS-EFX study period was lower at intervention sites than during the eSPEED trial several years prior: 11.3% vs 68.9%.
Discussion
In this retrospective cohort study of 21 community-based US EDs, more than a quarter of adults with acute PE were safely discharged home after a short ED stay. During a trial conducted 4 years earlier, intervention EDs had increased outpatient management with onsite champion promotion of decision support, without an increase at control sites. The practice gap was evident 4 years later, as intervention sites continued to have increased frequency of safe outpatient management of PE among patients with lower risk, concordant with CDSS recommendations.
Posttrial interventions were similar between ED groups. However, the eSPEED trial had 2 early performance advantages that may have put intervention EDs on a different trajectory, facilitating a sustained practice gap in managing PE among patients with lower risk.7 First, intervention physicians had extensive early training in decision support during the eSPEED trial. Use of the tool in 69% of eligible cases helped train them in risk stratification and determination of outpatient eligibility. Early use of PE risk stratification tools may have helped internalize PE risk stratification skills, making recourse to decision support over time less necessary (hence the lower recent use rate of 11.3% at intervention sites). We had surmised as much in a prior letter: “Such evidence-based cognitive education [via CDSS use] might equip users to function without dependency on the very rules that had earlier trained their judgment.”62 Tool-guided training in evidence-based site-of-care decision-making may help develop a more reliable gestalt. Even with decreasing dependency on decision support at intervention sites, decision support use remained higher 4 years later than at controls: 11.3% vs 7.4% (P = .03).
The second performance advantage was ongoing social influence: physicians at intervention sites were exposed to local social influence by onsite champions. Advocacy by champions for new practice patterns, especially if adopted by other department peers, may have helped to create a new culture of practice, establishing new local norms of behavior, which can be transforming and long-lasting.48,49,50,51 Interfacility differences in degrees of cultural transformation also might have contributed to the wide facility-specific variation we observed within our own health system, variation others have also reported across the country.15
It is unclear whether other US EDs also experienced as sizable an increase in outpatient management as we did. ED PE studies from earlier periods (2002 to 2013) have reported mixed results.63,64,65,66 A more recent, broadly representative site-of-care study across 740 US EDs in 2016 to 2018 found a low frequency of outpatient management (4.1%) but did not report time trends.15 A large US study of claims data found that outpatient management of PE among insured patients increased from 2011 to 2018 (16% to 23%).67 This attests to changing practice patterns in some organizations, but the results from this study cannot be directly compared with our own (eAppendix 9 in the Supplement).
Limitations
This study has some limitations. Our case ascertainment was incomplete, eg, we were unable to identify all patients with a clinical diagnosis of PE, such as those who lacked confirmatory pulmonary imaging. Fortunately, this population is small and likely affected intervention and control EDs equally.56 It is unclear which posttrial interventions may have been most directly associated with the systemwide increase in outpatient management. Given the observational study design, we cannot infer a causal relationship between posttrial interventions and physician site-of-care decision-making and cannot rule out unmeasured confounding. Incomplete documentation and missing variables that commonly beset retrospective research were mitigated regarding our outcomes, which were structured variables, reliably captured in our comprehensive EHR and not liable to be biased between ED sites. Our inability to capture unstructured documentation of mental status may have led to limited misclassification of the lower-risk cohort. However, in the eSPEED trial, altered mental status was identified in only 5% of patients, with similar rates between control and intervention sites.7 Additionally, our findings may not be generalizable to different populations and health care settings, especially those with less comprehensive or reliable follow-up infrastructure. However, the principal interventions we used—use of validated risk-stratification tools to provide point-of-care clinical decision support as well as enlisting onsite champions to educate, promote, and model practice change—can be readily adapted to a variety of practice settings.
Conclusions
This cohort study found that prior gains in outpatient management of acute PE among patients in the ED fostered by champion-led CDSS promotion were associated with increased outpatient management of PE among patients with lower risk 4 years after trial cessation. Insights from the SUS-EFX study will inform how we roll out new EHR-embedded decision support for other clinical conditions, now with an eye toward sustainability without continued promotion. How early high uptake of risk-stratification tools and social influence of embedded clinical champions may contribute to the long-term sustainability of practice-change interventions for other clinical conditions warrants further study.
eAppendix 1. Anticoagulant Recommendations During the SUS-EFX Study Period
eAppendix 2. Importance of Ready Access to Pharmacotherapy and Close Follow-up
eTable 1. Pulmonary Embolism Severity Index
eFigure. Example of Relative Contraindications to Outpatient Management of Emergency Department Patients With Acute Pulmonary Embolism Included in Electronic Clinical Decision Support System
eAppendix 3. Site-of-Care Recommendations
eAppendix 4. Method of Audit and Feedback Used in the eSPEED Trial
eAppendix 5. Altered Mental Status Variable of the Pulmonary Embolism Severity Index
eAppendix 6. Selective Manual Electronic Health Record Review
eAppendix 7. Defining 7-Day Pulmonary Embolism–Related Hospitalization for Those Managed as Outpatients
eTable 2. Emergency Department Patients With Acute Pulmonary Embolism Stratified by Direct Discharge Home vs Admission to an Observation Unit or Hospital
eTable 3. Characteristics and Course of Emergency Department Patients With Acute Pulmonary Embolism Discharged Directly Home Who Subsequently Experienced 7-Day Hospitalization for Pulmonary Embolism–Related Concerns or 30-Day All-Cause Mortality
eAppendix 8. Thirty-Day All-Cause Mortality of Emergency Department Patients With Acute Pulmonary Embolism
eTable 4. Thirty-Day All-Cause Mortality of Emergency Department Patients With Acute Pulmonary Embolism Stratified by the Pulmonary Embolism Severity Index Classification
eAppendix 9. A Large US Study of Claims Data
eReferences
References
- 1.Vinson DR, Hofmann ER, Johnson EJ, et al. ; PEPC Investigators of the KP CREST Network . Management and outcomes of adults diagnosed with acute pulmonary embolism in primary care: community-based retrospective cohort study. J Gen Intern Med. Published online January 12, 2022. doi: 10.1007/s11606-021-07289-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roy PM, Penaloza A, Hugli O, et al. ; HOME-PE Study Group . Triaging acute pulmonary embolism for home treatment by Hestia or simplified PESI criteria: the HOME-PE randomized trial. Eur Heart J. 2021;42(33):3146-3157. doi: 10.1093/eurheartj/ehab373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barco S, Schmidtmann I, Ageno W, et al. ; HoT-PE Investigators . Early discharge and home treatment of patients with low-risk pulmonary embolism with the oral factor Xa inhibitor rivaroxaban: an international multicentre single-arm clinical trial. Eur Heart J. 2020;41(4):509-518. doi: 10.1093/eurheartj/ehz367 [DOI] [PubMed] [Google Scholar]
- 4.Kabrhel C, Rosovsky R, Baugh C, et al. Multicenter implementation of a novel management protocol increases the outpatient treatment of pulmonary embolism and deep vein thrombosis. Acad Emerg Med. 2019;26(6):657-669. doi: 10.1111/acem.13640 [DOI] [PubMed] [Google Scholar]
- 5.Peacock WF, Singer AJ. Reducing the hospital burden associated with the treatment of pulmonary embolism. J Thromb Haemost. 2019;17(5):720-736. doi: 10.1111/jth.14423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bledsoe JR, Woller SC, Stevens SM, et al. Management of low-risk pulmonary embolism patients without hospitalization: the Low-Risk Pulmonary Embolism Prospective Management Study. Chest. 2018;154(2):249-256. doi: 10.1016/j.chest.2018.01.035 [DOI] [PubMed] [Google Scholar]
- 7.Vinson DR, Mark DG, Chettipally UK, et al. ; eSPEED Investigators of the KP CREST Network . Increasing safe outpatient management of emergency department patients with pulmonary embolism: a controlled pragmatic trial. Ann Intern Med. 2018;169(12):855-865. doi: 10.7326/M18-1206 [DOI] [PubMed] [Google Scholar]
- 8.Aujesky D, Roy PM, Verschuren F, et al. Outpatient versus inpatient treatment for patients with acute pulmonary embolism: an international, open-label, randomised, non-inferiority trial. Lancet. 2011;378(9785):41-48. doi: 10.1016/S0140-6736(11)60824-6 [DOI] [PubMed] [Google Scholar]
- 9.Roy PM, Corsi DJ, Carrier M, et al. Net clinical benefit of hospitalization versus outpatient management of patients with acute pulmonary embolism. J Thromb Haemost. 2017;15(4):685-694. doi: 10.1111/jth.13629 [DOI] [PubMed] [Google Scholar]
- 10.Dalen JE, Dalen JE Jr. Unnecessary hospitalizations for pulmonary embolism: impact on US health care costs. Am J Med. 2016;129(9):899-900. doi: 10.1016/j.amjmed.2016.03.041 [DOI] [PubMed] [Google Scholar]
- 11.Stevens SM, Woller SC, Baumann Kreuziger L, et al. Executive summary: antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160(6):2247-2259. doi: 10.1016/j.chest.2021.07.056 [DOI] [PubMed] [Google Scholar]
- 12.Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160(6):e545-e608. doi: 10.1016/j.chest.2021.07.055 [DOI] [PubMed] [Google Scholar]
- 13.Konstantinides SV, Meyer G, Becattini C, et al. ; ESC Scientific Document Group . 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41(4):543-603. doi: 10.1093/eurheartj/ehz405 [DOI] [PubMed] [Google Scholar]
- 14.Howard LSGE, Barden S, Condliffe R, et al. British Thoracic Society guideline for the initial outpatient management of pulmonary embolism (PE). Thorax. 2018;73(suppl 2):ii1-ii29. doi: 10.1136/thoraxjnl-2018-211539 [DOI] [PubMed] [Google Scholar]
- 15.Westafer LM, Shieh MS, Pekow PS, Stefan MS, Lindenauer PK. Outpatient management of patients following diagnosis of acute pulmonary embolism. Acad Emerg Med. 2021;28(3):336-345. doi: 10.1111/acem.14181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kline JA, Kahler ZP, Beam DM. Outpatient treatment of low-risk venous thromboembolism with monotherapy oral anticoagulation: patient quality of life outcomes and clinician acceptance. Patient Prefer Adherence. 2016;10:561-569. doi: 10.2147/PPA.S104446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patterson BW, Pulia MS, Ravi S, et al. Scope and influence of electronic health record-integrated clinical decision support in the emergency department: a systematic review. Ann Emerg Med. 2019;74(2):285-296. doi: 10.1016/j.annemergmed.2018.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwan JL, Lo L, Ferguson J, et al. Computerised clinical decision support systems and absolute improvements in care: meta-analysis of controlled clinical trials. BMJ. 2020;370:m3216. doi: 10.1136/bmj.m3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bledsoe JR, Kelly C, Stevens SM, et al. Electronic pulmonary embolism clinical decision support and effect on yield of computerized tomographic pulmonary angiography: ePE-A pragmatic prospective cohort study. J Am Coll Emerg Physicians Open. 2021;2(4):e12488. doi: 10.1002/emp2.12488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Urquhart R, Kendell C, Cornelissen E, et al. Defining sustainability in practice: views from implementing real-world innovations in health care. BMC Health Serv Res. 2020;20(1):87. doi: 10.1186/s12913-020-4933-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore JE, Mascarenhas A, Bain J, Straus SE. Developing a comprehensive definition of sustainability. Implement Sci. 2017;12(1):110. doi: 10.1186/s13012-017-0637-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon N, Lin T. The Kaiser Permanente Northern California adult member health survey. Perm J. 2016;20(4):15-225. doi: 10.7812/TPP/15-225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vinson DR, Ballard DW, Huang J, Rauchwerger AS, Reed ME, Mark DG; Kaiser Permanente CREST Network . Timing of discharge follow-up for acute pulmonary embolism: retrospective cohort study. West J Emerg Med. 2015;16(1):55-61. doi: 10.5811/westjem.2014.12.23310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.An J, Niu F, Zheng C, et al. Warfarin management and outcomes in patients with nonvalvular atrial fibrillation within an integrated health care system. J Manag Care Spec Pharm. 2017;23(6):700-712. doi: 10.18553/jmcp.2017.23.6.700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sylvester KW, Ting C, Lewin A, et al. Expanding anticoagulation management services to include direct oral anticoagulants. J Thromb Thrombolysis. 2018;45(2):274-280. doi: 10.1007/s11239-017-1602-1 [DOI] [PubMed] [Google Scholar]
- 26.Clark NP. Role of the anticoagulant monitoring service in 2018: beyond warfarin. Hematology Am Soc Hematol Educ Program. 2018;2018(1):348-352. doi: 10.1182/asheducation-2018.1.348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lobach D, Sanders GD, Bright TJ, et al. Enabling health care decisionmaking through clinical decision support and knowledge management. Evid Rep Technol Assess (Full Rep). 2012;(203):1-784. [PMC free article] [PubMed] [Google Scholar]
- 28.Sheehan B, Nigrovic LE, Dayan PS, et al. ; Pediatric Emergency Care Applied Research Network (PECARN) . Informing the design of clinical decision support services for evaluation of children with minor blunt head trauma in the emergency department: a sociotechnical analysis. J Biomed Inform. 2013;46(5):905-913. doi: 10.1016/j.jbi.2013.07.005 [DOI] [PubMed] [Google Scholar]
- 29.Miller P, Phipps M, Chatterjee S, et al. Exploring a clinically friendly web-based approach to clinical decision support linked to the electronic health record: design philosophy, prototype implementation, and framework for assessment. JMIR Med Inform. 2014;2(2):e20. doi: 10.2196/medinform.3586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trinkley KE, Kahn MG, Bennett TD, et al. Integrating the practical robust implementation and sustainability model with best practices in clinical decision support design: implementation science approach. J Med Internet Res. 2020;22(10):e19676. doi: 10.2196/19676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172(8):1041-1046. doi: 10.1164/rccm.200506-862OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vinson DR, Morley JE, Huang J, et al. ; Kaisers Permanente CREST Network . The accuracy of an electronic Pulmonary Embolism Severity Index auto-populated from the electronic health record: setting the stage for computerized clinical decision support. Appl Clin Inform. 2015;6(2):318-333. doi: 10.4338/ACI-2014-12-RA-0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vinson DR, Aujesky D, Geersing GJ, Roy PM. Comprehensive outpatient management of low-risk pulmonary embolism: can primary care do this—a narrative review. Perm J. 2020;24:19. doi: 10.7812/TPP/19.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zondag W, Mos IC, Creemers-Schild D, et al. ; Hestia Study Investigators . Outpatient treatment in patients with acute pulmonary embolism: the Hestia Study. J Thromb Haemost. 2011;9(8):1500-1507. doi: 10.1111/j.1538-7836.2011.04388.x [DOI] [PubMed] [Google Scholar]
- 35.Erkens PM, Gandara E, Wells P, et al. Safety of outpatient treatment in acute pulmonary embolism. J Thromb Haemost. 2010;8(11):2412-2417. doi: 10.1111/j.1538-7836.2010.04041.x [DOI] [PubMed] [Google Scholar]
- 36.Vinson DR, Mark DG, Ballard DW. Outpatient management of patients with pulmonary embolism. Ann Intern Med. 2019;171(3):228. doi: 10.7326/L19-0208 [DOI] [PubMed] [Google Scholar]
- 37.Becattini C, Maraziti G, Vinson DR, et al. Right ventricle assessment in patients with pulmonary embolism at low risk for death based on clinical models: an individual patient data meta-analysis. Eur Heart J. 2021;42(33):3190-3199. doi: 10.1093/eurheartj/ehab329 [DOI] [PubMed] [Google Scholar]
- 38.Arrigo M, Huber LC. Right ventricle assessment in patients with pulmonary embolism: low risk = low yield for systematic echocardiography. Eur Heart J. 2021;43(1):84-85. doi: 10.1093/eurheartj/ehab762 [DOI] [PubMed] [Google Scholar]
- 39.Maraziti G, Vinson DR, Becattini C. Echocardiography for risk stratification in patients with pulmonary embolism at low risk of death: a response. Eur Heart J. 2021;43(1):86-87. doi: 10.1093/eurheartj/ehab779 [DOI] [PubMed] [Google Scholar]
- 40.Raper JD, Thomas AM, Lupez K, et al. Can right ventricular assessments improve triaging of low risk pulmonary embolism? Acad Emerg Med. Published online March 15, 2022. doi: 10.1111/acem.14484 [DOI] [PubMed] [Google Scholar]
- 41.Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352. doi: 10.1016/j.chest.2015.11.026 [DOI] [PubMed] [Google Scholar]
- 42.Mostofian F, Ruban C, Simunovic N, Bhandari M. Changing physician behavior: what works? Am J Manag Care. 2015;21(1):75-84. [PubMed] [Google Scholar]
- 43.Gulliford MC, Prevost AT, Charlton J, et al. Effectiveness and safety of electronically delivered prescribing feedback and decision support on antibiotic use for respiratory illness in primary care: REDUCE cluster randomised trial. BMJ. 2019;364:l236. doi: 10.1136/bmj.l236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moja L, Liberati EG, Galuppo L, et al. Barriers and facilitators to the uptake of computerized clinical decision support systems in specialty hospitals: protocol for a qualitative cross-sectional study. Implement Sci. 2014;9:105. doi: 10.1186/s13012-014-0105-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shan J, Isaacs DJ, Bath H, Johnson EJ, Julien D, Vinson DR. “Outpatient management” of pulmonary embolism defined in the primary literature: a narrative review. Perm J. 2021;25:20-303. doi: 10.7812/TPP/20.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allen C, Coleman K, Mettert K, Lewis C, Westbrook E, Lozano P. A roadmap to operationalize and evaluate impact in a learning health system. Learn Health Syst. 2021;5(4):e10258. doi: 10.1002/lrh2.10258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Osuji TA, Frantsve-Hawley J, Jolles MP, Kitzman H, Parry C, Gould MK; Embedded Research Conference Priorities and Methods Workgroup . Methods to identify and prioritize research projects and perform embedded research in learning healthcare systems. Healthc (Amst). 2020;8(4):100476. doi: 10.1016/j.hjdsi.2020.100476 [DOI] [PubMed] [Google Scholar]
- 48.Keating NL, O’Malley AJ, Onnela J-P, Gray SW, Landon BE. Association of physician peer influence with subsequent physician adoption and use of bevacizumab. JAMA Netw Open. 2020;3(1):e1918586. doi: 10.1001/jamanetworkopen.2019.18586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Centola D. Physician networks and the complex contagion of clinical treatment. JAMA Netw Open. 2020;3(1):e1918585. doi: 10.1001/jamanetworkopen.2019.18585 [DOI] [PubMed] [Google Scholar]
- 50.Pollack CE, Soulos PR, Herrin J, et al. The impact of social contagion on physician adoption of advanced imaging tests in breast cancer. J Natl Cancer Inst. 2017;109(8):djw330. doi: 10.1093/jnci/djw330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keating NL. Peer influence and opportunities for physician behavior change. J Natl Cancer Inst. 2017;109(8):djx009. doi: 10.1093/jnci/djx009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vinson DR, Drenten CE, Huang J, et al. ; Kaiser Permanente Clinical Research on Emergency Services and Treatment (CREST) Network . Impact of relative contraindications to home management in emergency department patients with low-risk pulmonary embolism. Ann Am Thorac Soc. 2015;12(5):666-673. doi: 10.1513/AnnalsATS.201411-548OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ballard DW, Vemula R, Chettipally UK, et al. ; KP CREST Network Investigators . Optimizing clinical decision support in the electronic health record: clinical characteristics associated with the use of a decision tool for disposition of ED patients with pulmonary embolism. Appl Clin Inform. 2016;7(3):883-898. doi: 10.4338/ACI-2016-05-RA-0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vinson DR, Ballard DW, Mark DG, et al. ; MAPLE investigators of the KP CREST Network . Risk stratifying emergency department patients with acute pulmonary embolism: does the simplified Pulmonary Embolism Severity Index perform as well as the original? Thromb Res. 2016;148:1-8. doi: 10.1016/j.thromres.2016.09.023 [DOI] [PubMed] [Google Scholar]
- 55.Simon LE, Iskin HR, Vemula R, et al. Emergency department patient satisfaction with treatment of low-risk pulmonary embolism. West J Emerg Med. 2018;19(6):938-946. doi: 10.5811/westjem.2018.9.38865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vinson DR, Ballard DW, Huang J, et al. ; MAPLE Investigators of the KP CREST Network . Outpatient management of emergency department patients with acute pulmonary embolism: variation, patient characteristics, and outcomes. Ann Emerg Med. 2018;72(1):62-72.e3. doi: 10.1016/j.annemergmed.2017.10.022 [DOI] [PubMed] [Google Scholar]
- 57.Vinson DR, Mark DG, Ballard DW. Overcoming barriers to outpatient management of emergency department patients with acute pulmonary embolism. Acad Emerg Med. 2021;28(3):377-378. doi: 10.1111/acem.14210 [DOI] [PubMed] [Google Scholar]
- 58.Stein PD, Matta F, Hughes PG, et al. Home treatment of pulmonary embolism in the era of novel oral anticoagulants. Am J Med. 2016;129(9):974-977. doi: 10.1016/j.amjmed.2016.03.035 [DOI] [PubMed] [Google Scholar]
- 59.Aujesky D, Roy PM, Le Manach CP, et al. Validation of a model to predict adverse outcomes in patients with pulmonary embolism. Eur Heart J. 2006;27(4):476-481. doi: 10.1093/eurheartj/ehi588 [DOI] [PubMed] [Google Scholar]
- 60.Aujesky D, Perrier A, Roy PM, et al. Validation of a clinical prognostic model to identify low-risk patients with pulmonary embolism. J Intern Med. 2007;261(6):597-604. doi: 10.1111/j.1365-2796.2007.01785.x [DOI] [PubMed] [Google Scholar]
- 61.Vinson DR, Bath H, Huang J, Reed ME, Mark DG; CREST Network . Hospitalization is less common in ambulatory patients with acute pulmonary embolism diagnosed before emergency department referral than after arrival. Acad Emerg Med. 2020;27(7):588-599. doi: 10.1111/acem.14034 [DOI] [PubMed] [Google Scholar]
- 62.Vinson DR, Ballard DW, Dayan PS, Kuppermann N. Clinical judgment and the pediatric emergency care applied research network head trauma prediction rules. Ann Emerg Med. 2018;72(3):323-325. doi: 10.1016/j.annemergmed.2018.04.005 [DOI] [PubMed] [Google Scholar]
- 63.Smith SB, Geske JB, Kathuria P, et al. Analysis of national trends in admissions for pulmonary embolism. Chest. 2016;150(1):35-45. doi: 10.1016/j.chest.2016.02.638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brahmandam A, Abougergi MS, Ochoa Chaar CI. National trends in hospitalizations for venous thromboembolism. J Vasc Surg Venous Lymphat Disord. 2017;5(5):621-629.e2. doi: 10.1016/j.jvsv.2017.04.006 [DOI] [PubMed] [Google Scholar]
- 65.Stein PD, Matta F, Hughes MJ. National trends in home treatment of acute pulmonary embolism. Clin Appl Thromb Hemost. 2018;24(1):115-121. doi: 10.1177/1076029616674827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fang MC, Fan D, Sung SH, et al. Outcomes in adults with acute pulmonary embolism who are discharged from emergency departments: the Cardiovascular Research Network Venous Thromboembolism study. JAMA Intern Med. 2015;175(6):1060-1062. doi: 10.1001/jamainternmed.2015.0936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lutsey PL, Walker RF, MacLehose RF, et al. Inpatient versus outpatient acute venous thromboembolism management: trends and postacute healthcare utilization from 2011 to 2018. J Am Heart Assoc. 2021;10(20):e020428. doi: 10.1161/JAHA.120.020428 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Anticoagulant Recommendations During the SUS-EFX Study Period
eAppendix 2. Importance of Ready Access to Pharmacotherapy and Close Follow-up
eTable 1. Pulmonary Embolism Severity Index
eFigure. Example of Relative Contraindications to Outpatient Management of Emergency Department Patients With Acute Pulmonary Embolism Included in Electronic Clinical Decision Support System
eAppendix 3. Site-of-Care Recommendations
eAppendix 4. Method of Audit and Feedback Used in the eSPEED Trial
eAppendix 5. Altered Mental Status Variable of the Pulmonary Embolism Severity Index
eAppendix 6. Selective Manual Electronic Health Record Review
eAppendix 7. Defining 7-Day Pulmonary Embolism–Related Hospitalization for Those Managed as Outpatients
eTable 2. Emergency Department Patients With Acute Pulmonary Embolism Stratified by Direct Discharge Home vs Admission to an Observation Unit or Hospital
eTable 3. Characteristics and Course of Emergency Department Patients With Acute Pulmonary Embolism Discharged Directly Home Who Subsequently Experienced 7-Day Hospitalization for Pulmonary Embolism–Related Concerns or 30-Day All-Cause Mortality
eAppendix 8. Thirty-Day All-Cause Mortality of Emergency Department Patients With Acute Pulmonary Embolism
eTable 4. Thirty-Day All-Cause Mortality of Emergency Department Patients With Acute Pulmonary Embolism Stratified by the Pulmonary Embolism Severity Index Classification
eAppendix 9. A Large US Study of Claims Data
eReferences