Abstract
Phlorotannins, a seaweed based class of polyphenolic compounds, have proven to possess potential bioactivities such as antioxidant, antimicrobial, anti-allergic, anti-diabetic, anti-inflammatory, anti-cancerous, neuroprotection etc. These bioactivities have further increased demand globally and sustainable techniques such as supercritical fluid extraction, microwave assisted extraction, enzyme assisted extraction, extraction using deep eutectic solvents etc. are being explored currently for production of phlorotannin-rich extracts. In spite of such well documented bioactivities, very few phlorotannin-based nutraceuticals are available commercially which highlights the significance of generating consumer awareness about their physiological benefits. However, for industry level commercialization accurate quantification of phlorotannins with respect to the different classes is vital requiring sophisticated analytical techniques such as mass spectrometry, 1H-NMR spectroscopy etc. owing to the wide structural diversity. This review summarizes the extraction and bioactivities of phlorotannins based on the findings of in vivo and in vitro studies.
Keywords: Phloroethols, Neurodegeneration, Melanosis, Histamine, Hyaluronidase, Supercritical fluid extraction, Nitric oxide, Lipopolysaccharide, Anti-viral, Anti-proliferative
Introduction
The prospects of marine ecosystems as a repository of bioactive compounds is gaining increasing momentum. Seaweeds, one of the largest biomass producers of marine environment, have been included in the diet as well as traditional medicine for centuries (Fleurence & Levine 2016). It has been estimated (Guiry 2014) that there are about 10, 000 seaweed species globally. Earlier, seaweeds were used mainly in preparation of livestock diets because of their rich nutrient composition, however gradually the focus has shifted to the development of drugs and nutraceuticals for human consumption. It has been reported that there is an ever increasing demand for seaweed globally with an anticipation of its value increasing up to US$ 26 million by 2025 (Ferdouse et al. 2018). Seaweeds are classified into three classes: Brown (Phaeophyceae), red (Rhodophyceae) and green (Chlorophyceae) based on their pigmentation. Among all, brown seaweeds are reported to be the richest in terms of their structural diversity. Balboa et al. (2013) reported that brown seaweeds have comparatively higher antioxidant potential than red and green algae. Generally, seaweeds are reported to be rich repository of bioactive compounds such as sulphated polysaccharides, carotenoids, phytosterols, phycobiliproteins, phloroglucinol etc. The presence of polysaccharides such as alginate, laminarin and sulphated fucoidan etc. make them nutritionally significant from other seaweeds. Of late, there is an increasing trend among researchers to exploit seaweeds as source of natural antioxidants. The bioactive compounds in seaweeds such as fucoidan, fucoxanthin, phlorotannins, are known for their antioxidative activities.
Phlorotannins
Phlorotannins constitute an important class of polyphenolic compounds in marine brown algae accounting for about 5 -12% of their dry weight (Venkatesan et al. 2019). Phlorotannins, which are structurally 1,3,5-trihydroxybenzene, are formed by the polymerization of phloroglucinol units. Basically, they are of hydrophilic in nature with molecular weight ranging from 126 Da–650 kDa (Barbosa et al. 2017). Depending on the degree of polymerization and structural diversities, phlorotannins can be categorized into six different classes such as phloroethols, fuhalols, fucophloroethols, fucols, eckols and carmalols. In phloroethols, the monomers (phloroglucinol) are connected via an ether bond. Similarly, in case of fuhalols, an ether linkage interconnects the phloroglucinol units, however an additional hydroxyl group is present at every third ring. Fucophlorethols possess both ether and phenyl bonds. In case of fucols, phenyl linkage interconnects the monomer units where as in eckols, 1,4-dibenzodioxin is present in the structure. Carmalols are characterized by a4-dibenzodioxin linkage at the 3rd and 7th position.
Being secondary metabolites they are mainly produced by algae as a defense mechanism to survive in the challenging aquatic conditions. Hence, their content can also vary with the changes in environmental conditions. The available scientific literature points out the structural diversity of phlorotannins which arises due to several factors such as age and size of algae, environmental factors in which the species prevails etc. especially, the temperature, salinity, incidence of solar radiation etc. Kirke et al. (2019) have studied the temporal variation in low molecular weight phlorotannin fractions content in certain intertidal algae. It was observed that the low molecular weight phlorotannin profile are dependent on the type of species and only minor effects were induced by external factors in modulating its profile.
Phlorotannins display a wide range of bioactivities such as antioxidant, antimicrobial, anticancerous, anti-inflammatory, anti-diabetic, protection from UV radiation etc. It is reported to inhibit the reactive oxygen species and thiobarbituric acid reactive substances production indicating the antioxidant properties. Recently, cold plasma treatment has been employed to produce polymeric phlorotannins to broaden radical scavenging and antimicrobial activities. Kang et al. (2013) reported the protective effect of phlorotannins against oxidative stress based on a zebra fish embryo model study and stated that they were not toxic at the tested concentrations indicating their efficacy and safety. The increasing scientific evidence regarding their bioactivities has further fostered their extraction to use in various sectors. In addition to the conventional extraction methods, several sustainable techniques are currently being used widely for obtaining phlorotannins from brown seaweeds.
Broader applications of phlorotannins
Anti-allergic/histamine activity
Phlorotannins demonstrate a range of biological activities such as antioxidant, antimicrobial, antiviral, anticancer, anti-inflammatory, anti-diabetic, neuroprotective activity etc. (Fig. 1, Table 1.). Allergic conditions are among the most prevalent immunological diseases globally. Among the several approaches to control allergic diseases, decreasing the production of immunoglobulin E (IgE), inhibiting the production of mediators and their subsequent release from mast cells are the most common. Recently there has been a rise in the search for natural based anti-allergic compounds to control such immunological disorders. Seaweed based class of polyphenolic compounds such as phlorotannins are reported to demonstrate potential anti-allergic activities.
Fig. 1.
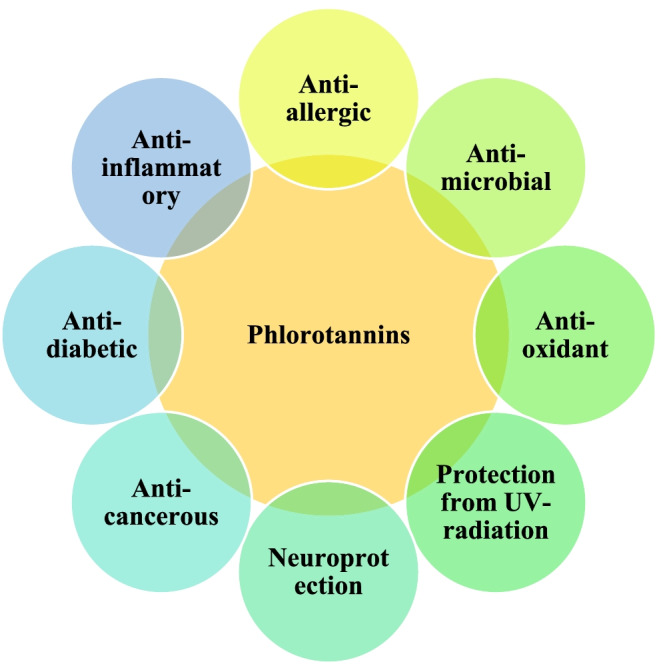
Bioactivities of phlorotannins
Table 1.
Bioactivities of Phlorotannins
| Sl No: | Bioactivity | References | Significant findings |
|---|---|---|---|
| 1 | Anti-allergic effects | Matsui et al. (2022) | Fuhalol class of phlorotannins exhibited multiple anti allergic effects |
| 2 | Neuroprotection | Shrestha et al (2021) | Phlorotannin compound identified as dibenzodioxin-fucodiphloroethol exhibits significant neuroprotection against amyloid β neurotoxicity suggesting it as suitable candidate for treatment of Alzheimer’s disease |
| 3 | Prebiotic effect | Vázquez-Rodríguez et al (2021) | Phlorotannin along with mannitol is reported to augment the growth of Bifidobacterium and Lactobacillus suggesting its potential in improving the human gut microbiota |
| 4 | Biopreservative effect | Sharifian et al (2019) | Ice storage study of shrimps treated with phlorotannins demonstrated their activity against polyphenol oxidase activity suggesting a role as a natural melanosis control agent. Further, the overall quality improvement in terms of chemical, sensory and microbiological activity was also demonstrated by application of phlorotannins |
| 5 | Biopreservative effect | Yu et al. (2022) | The physical and microbial quality of refrigerated seabass fillets were found to be improved by application of phlorotannins immobilised nanochitin. Further, treatment has helped in attaining a shelf life extension by 3 days suggesting its potential as a biopreservative agent |
| 5 | Fucoidanase inhibitory effect | Imbs et al (2018) | Phlorotannins extracted from brown algae Fucus evanescens and Costaria costata were of high molecular weight mostly belonging to fucophloretol and phlorethols respectively. The extracts showed fucoidanase inhibitory effect and the activity was highly related to molecular weight. The nature of inhibition was reported to be irreversible |
| 6 | Anti-cancer activity | Abdelhamid et al (2019) | Phlorotannins extracted from Cystoseira sedoides exhibited potential apoptotic effect towards MCF-7 breast cancer cell line and their activities were found to be dose dependent |
| 7 | Cosmeceutical activity | Ferreira et al (2021) | Electrospun fibre based wound dressing material with phlorotannin-rich extracts were found to have better stability and lesser degradation rate suggesting its potential in wound healing and skin regeneration |
| 8 | Nutraceutical application | Bai et al. (2022) | Phlorotannins encapsulated in Whey protein-chitosan oligosaccharides exhibited better antioxidant, anti-inflammatory and solubility attributes suggesting its use as a potent nutraceutical |
| 9 | Nutraceutical application | Wang et al (2021) | Phlorotannin extracts prepared from Laminaria japonica when used in conjuction with UV radiation treatment were found to improve myofibrillar protein gel quality favored by non-covalent and covalent protein linking interactions |
| 10 | Prebiotic effect | Catarino et al (2021) | Phlorotannin extracts prepared from Fucus vesiculosus positive implications in modulating the human gut microbiota |
Le et al. (2009) extracted and purified phlorotannins from Ecklonia cava and studied anti-allergenicity by quantifying the amount of histamine and β-hexosaminidase release on human basophilic leukemia (KU812F) and rat basophilic leukemia (RBL-2H3) cell lines, respectively. They reported that dieckol showed the highest inhibitory activity on KU812 cells and 6,6’-bieckol exerted most effective inhibition on the degranulation level of RBL-2H3 cells. They also reported the bioactivity of phlorotannins is strongly related to molecular size or the number of phenol groups. Ability of phlorotannins to inhibit histamine release was also reported and correlated with their ability to stabilize the cell membrane by reducing the level of intracellular Ca2+ levels. Similarly, the anti-allergic activities of phlorotannins, specifically fucodiphloroethol and phlorofucofuroeckol, obtained from E. cava on human basophilic leukemia (KU812) and rat basophilic leukemia (RBL-2H3) were reported by Li et al. (2008).
Ahn et al. (2015) reported that dieckol, obtained from E. cava, decreased the release of β‐hexosaminidase and histamine in antigen‐stimulated bone marrow‐derived cultured mast cells. However, the inhibitory effects were dose dependent. Flow cytometry analysis indicated that dieckol significantly reduced the expression of high-affinity IgE receptors (FcεRI). The outcomes of the study highlighted the possibilities of dieckol application in treatment of allergic diseases. Sugiura et al. (2013) studied the anti-allergic and anti-inflammatory effects of phlorotannins obtained from the brown alga Eisenia arborea by in vivo studies in ICR mice. Ear edema was induced by three compounds, arachidonate, 12-O-tetradecanoylphorbol-13-acetate (TPA) and oxazolone and the inhibition effects of phlorotannin of two different concentrations, 0.01 mg, 0.1 mg, were studied by keeping epigallocatechin gallate (EGCG) as the natural inhibitor. The inhibitory effects of phlorotannins were found to be comparable with that of EGCG. However, the tested concentration was more effective for inhibiting edema induced by arachidonate and oxazolone than TPA. The authors reported that the concentration of phlorotannins employed in the study was more effective in suppressing the expression of lipoxygenase gene than cyclooxygenase-2 gene. The study proposed that phlorotannin compounds can be suggested as a natural inhibitor for delaying type IV allergic reactions.
Barbosa et al. (2017) have purified phlorotannin-rich extracts from Fucus sp. using aqueous acetone based extraction and subjected to anti allergenicity studies. The extracts were found to lower the rate of degranulation of RBL-2H3 cells and reduced the release of histamine and β-hexosaminidase to a greater extent. The authors have attributed the antiallergic mechanism to the binding modulation between immunoglobulin, IgE and high-affinity IgE receptors by virtue of possible formation of insoluble complexes between phlorotannins and potential allergens, making the extract hypoallergenic. Further, the role of phlorotannin in cell membrane stabilization by reducing the intracellular Ca2+ levels was also discussed. The extracts were also found to possess significant hyaluronidase activity.
Anti-microbial/anti-viral/anti-fungal activity
There is a growing interest in the use of natural compounds as safer alternatives to antibiotics, as antibiotic resistance is one of the global health concerns. Bioactive compounds in seaweeds are reported to exhibit potential antimicrobial/antiviral activity by several modes of action. Kwon et al. (2013) explored the anti-viral activity of phlorotannins extracted from E. cava against porcine epidemic diarrhea virus. It was hypothesized that phlorotannins exerted antiviral activity through two major pathways such as by blocking the entry of virus into cells or/and by inhibiting the viral replication after its entry into the host cell. Phlorofucofuroeckol and dieckol were the two major phlorotannins found in the extracts which are reported to have strong inhibition activities.
Severe acute respiratory syndrome coronavirus, known for its life threatening infection due to acute pneumonia has emerged as a global concern recently. Park et al. (2013) have reported that dieckol from E. cava has competitive inhibition activity towards 3CLpro, a chymotrypsin-like cysteine proteinase, which is essential for viral replication. Eom et al. (2013) studied the anti-bacterial activity of phlorotannins isolated from Eisenia bicyclis against methicillin-resistant Staphylococcus aureus (MRSA). Phlorotannins showed anti-bacterial activity against MRSA with phlorofucofuroeckol-A exhibiting highest activity. However, their effects were less when compared to commercial antibiotics. It was further demonstrated that phlorofucofuroeckol-A used in conjunction with β-lactam antibiotics had synergistic effects.
Lopes et al. (2013) found significant antifungal activity of phlorotannins extracted from brown seaweeds against yeast and several dermatophytes and the effects were attributed to their ability to reduce ergosterol content of the fungal cell wall. Phlorotannins have increased the activity of mitochondrial dehydrogenases causing membrane depolarization. Further, phlorotannins were demonstrated to have the potential to inhibit the dimorphic transition of Candida cells indicating their efficacy as an antifungal agent.
Kim et al. (2018) studied the anti-Listerial activity of phlorotannins isolated from E. bicyclis using disc diffusion and micro-dilution methods. Among the different phlorotannin compounds isolated, fucofuroeckol-A exhibited the highest anti-Listerial potential. When used in conjunction with the antibiotic streptomycin synergistic effects were observed. Yang et al. (2018) studied the anti-viral activity of phlorotannin rich extract obtained from E. cava against viral hemorrhagic septicemia virus in a fathead minnow (FHM) cell line. The plaque reduction assay revealed the higher inhibition effect of phlorotannin derivatives. Further, the challenge study of virus in olive flounder indicated that the extract induced pro-inflammatory cytokines and activated immune cells such as macrophages and natural killer cells. Potential antimicrobial/antiviral activity of phlorotannin indicates that it can used as biocontrol agents.
Cho et al. (2019) reported that phlorotannins isolated from E. cava have significant anti-viral activity by inhibiting the expression of surface proteins, viz, hemagglutinin, and neuraminidase. Nair et al. (2019) have reported the presence of fucophlorethol class of phlorotannins from Padina tetrastromatica extracts with degree of polymerization ranging from 2 to 18. It was observed that the antioxidant activity of phlorotannins is strongly related to the degree of polymerization. Further, they established its anti-inflammatory potential and significant activity against methicillin resistant Staphylococcus aureus.
Inhibitor of neurodegeneration
Kannan et al. (2013) extracted and purified phlorotannins from Ecklonia maxima using solvent extraction followed by partitioning. Structural elucidation by mass spectrometry indicated that the extracts comprised of 3 compounds: 1,3,5-trihydroxybenzene, dibenzo [1,4] dioxine-2,4,7,9-tetraol and eckol. The extracts exhibited significant acetylcholinesterase inhibitory activity and their effects dose-dependent. Findings of the study indicated that they potentially can be used in treatment of neurodegenerative diseases like Alzheimer’s.
Lee et al. (2019) investigated the neuroprotection property of phlorotannins isolated from E. cava using in vivo assays. Among the different class of phlorotannin compounds, dieckol had potent anti-apoptotic activity, attenuation activity towards PGE2 and in restoring mitochondrial membrane potential. In general, the anti –inflammatory activity of the tested phlorotannin class of compounds were attributed to the down regulation of specific enzymes, inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2).
Barbosa et al. (2020) reported the extraction of phlorotannins from edible Fucus sp using 70% acetone and analysed its bioactivity, especially its ability to interact with enzymatic systems which are connected to the onset and progression of neurodegeneration. On further characterization, the extracted phlorotannins were found to be of low degree of polymerization belonging to the classes of fucophloretols and eckols. The extracts were effective in inhibiting acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) and tyrosinase enzyme activities which are of significance in neurodegenerative diseases. The study underpinned the potential of phlorotannin-rich extracts in inhibiting the enzymatic systems that are involved in progression of neurodegeneration pointing out to their significant potential in neuroresearch.
Anti-inflammatory activity
Inflammation is the body’s basic defense mechanism to any foreign stimulus. The process of inflammation involves a number of inflammatory mediators such as lipoxygenases, leukotrienes, cytokines, platelet-activating factor, prostaglandins etc. which are produced by mast cells, macrophages and neutrophils. Anti-inflammatory agents are often employed to prevent the progression of inflammatory conditions and to maintain the tissue homeostasis (Bai et al. 2021). Recently, there is an increasing trend to use natural compounds as anti-inflammatory agents. Anti-inflammatory activity of phlorotannin compounds has been already well-established (Barbosa et al. 2019).
Kim et al. (2012) studied the protective effect of three classes of phlorotannin compounds such as phloroglucinol, eckol, and dieckol against the pro-inflammatory responses in human umbilical vein endothelial cells and in mice. The study highlighted that the phlorotannins helped in protecting the vascular barrier integrity and by adversely affecting the adhesion and migration of leukocytes. The potential of phlorotannins as an anti-inflammatory agent was emphasized in the study.
Wijesinghe et al. (2013) evaluated the anti-inflammatory potential of Candida utilis fermented Ecklonia cava by-product using in vitro assays using lipopolysaccharide activated murine macrophage cell line RAW 264.7. Phlorotannin-rich extracts exhibited significant reduction in nitric oxide production and the effects were reported to be dose dependent. Further, lactate dehydrogenase assay indicated that the extracts were not cytotoxic in nature indicated their safety. Extracts have shown significant inhibition effects against production of nitric oxide synthase, prostaglandin-E2 and cyclooxygenase-2 indicating their anti-inflammatory activity. Similarly, Jung et al. (2013) reported that phloroglucinol and phlorofucofuoeckol A (present in the ethanolic extracts of E. bicyclis) had potential anti-inflammatory activity. Both compounds had strong inhibition effect in production of nitric oxide in cellular systems.
Barbosa et al. (2017) employed acetone based extraction for obtaining phlorotannin-rich extracts from Fucus spp. and purified the same for further analysis. The purified extracts were screened for the anti-inflammatory potential of and biosafety. The extracts exhibited high radical scavenging activity and the effects were found to be dose dependent. Furthermore, the extracts shown significant inhibition against lipoxygenases, which are enzymes involved in synthesizing of mediators for progression of inflammation related diseases. The extracts were found to be non-toxic indicating their safety for use especially as anti-inflammatory agents.
Son et al. (2020) obtained phlorotannin-rich fractions from E. cava using conventional extraction with 50% aqueous ethanol as solvent. The phlorotannin-rich extracts were further evaluated for its effect against inflammation and leptin resistance in an animal model study. It was reported that the extracts attenuated the expression of receptors associated with inflammation and leptin resistance and thereby favoring lipolysis. The significant findings of the study highlighted the E. cava extracts potentially can be used in treatment of obesity, inflammation and related diseases.
Anti-diabetic activity
Diabetes mellitus, is reported to be one of the growing health disease globally. Type-2 diabetes, is often categorized as the most prevalent type of diabetes globally, characterized by hyperglycemia. Inhibitors of enzymes, such as α-amylase and α-glucosidase which are important for digestion and metabolism of carbohydrates, are employed for diabetes control. Recently, there has been a surge for natural products to be used for diabetes management owing to their lesser side effects. Kellogg et al (2014) reported the anti-diabetic potential of phlorotannins by virtue of their ability to inhibit carbohydrate hydrolyzing enzymes such as α-amylase, glucosidase.
Lee et al. (2016) have explored the anti-diabetic effects of octaphlorethol A, a phlorotannin compound isolated from Ishige foliacea based on an animal model study. It was reported that in case of animals treated with octaphlorethol A, there was a lowering in postprandial blood glucose and lower insulin levels. Further, glucose uptake was enhanced in octaphlorethol A fed group via the upregulated expression of glucose transporter 4. Hepatic glucose was also reported to be lowered by inhibiting enzymes such as glucose-6-phosphatase and phosphoenolpyruvate carboxykinase which are important for gluconeogenesis.
Park et al. (2018) extracted phlorotannins from E. cava using 80% aqueous alcohol and the minor phlorotannins obtained from the ethyl acetate fractions were screened for α-glucosidase inhibition activity. Certain compounds obtained from the ethyl acetate fractions exhibited significant inhibitory activity against α-glucosidase, an enzyme which is of significance in the treatment of type -2 diabetes. Ethyl acetate fractions were found to be non-competitive and competitive inhibitors too.
Lopes et al. (2019) extracted and purified phlorotannin rich extracts from different species of Fucus and determined the antidiabetic potential through several enzyme assays. The extracts were capable of inhibiting the activity of major carbohydrate hydrolyses such as α-amylase, glucosidase and the activity was mostly exhibited by extracts rich in fucophlorethol class of phlorotannins of lower molecular weight. The study highlighted that the bioactivity of phlorotannin compounds is highly correlated to its structure along with the molecular weight.
Gheda et al. (2021) extracted phlorotannins from Cystoseira compressa and on detailed structural elucidation, fuhalol was identified as the major phlorotannin compound in the extract. The phlorotannin-rich extract was reported to have significant antidiabetic potential as evident from the lowered serum glucose level and increased serum insulin level. Furthermore, phlorotannins inhibited α-amylase, glucosidase activities and helped in reducing damage of β cells of pancreases. The authors concluded that phlorotannins hold promising therapeutic potential for treatment of diabetes.
Hwang et al (2021) studied the effect of phloroglucinol and dieckol on inhibiting angiogenesis under high glucose conditions. In silico docking study revealed that both the compounds had strong binding affinity towards the antiangiogenic receptor,vascular endothelial growth factor VEFGR and thereby helped in inhibiting the endothelial cell proliferation, migration and vascular formation. Findings of this study suggest that they can be used as nutraceuticals having potential anti-angiogenic effects.
As bioactive agent in packaging films
Phlorotannins, by virtue of their antioxidant and antimicrobial potential, are finding applications in food industries especially in the preservation, packaging and nutraceutical sectors. Surendhiran et al (2019) attempted to develop a biodegradable antimicrobial packaging material nanofiber mat with encapsulated phlorotannins. It was observed that phlorotannin had significant antimicrobial activity against Staphylococcus enteritidis. Detailed analysis of mechanism of action of phlorotannin on S. enteritidis indicated that phlorotannins cause cellular death by affecting the physical and biological activity of the macromolecules such as ATP, DNA and protein. Apart from the antimicrobial activity, phlorotannin also exhibited significant antioxidant activity as evident from the better sensory quality of the product.
Similarly, Cui et al. (2020) have also reported the encapsulation of phlorotannin in Momordica charantia polysaccharides nanofibers for developing active food packaging material. The material developed was subjected to cold plasma for improved performance. Better loading and release of phlorotannin from the nanofibers were observed suggesting its use as potential antioxidant cum antimicrobial agents especially for the food industry.
UV-radiation protection
The UV protection role of phlorotannins has been studied well and this has fostered their use in cosmeceuticals. It has been reported that phlorotannins can absorb UV radiation, especially UV-C and partly UV-B, with maxima at 195 nm and 265 nm. In this aspect, Creis et al. (2015) have extracted and purified phlorotannins from Fucus vesiculosus. Structural elucidation by HPLC–ESI–MS showed that the extracts were comprised of phlorotannins with degree of polymerisation 3 to 7. The study investigated whether the accumulation of phenolic compounds is inducible or constitutive by exposing the algae to UV-B radiation. The exposure to UV-B radiation induced the over-expression of heat shock protein, which is reported to be involved in sulfation of phlorotannins. However, further results indicated that the accumulation of phlorotannins in F. vesiculosus occurs during its development process, suggesting that the process is constitutive.
Anti-cancer activity
Yang et al. (2015) studied whether phlorotannin rich extracts from E. cava can positively modulate the tumor growth inhibitory effect of cisplatin by in vivo assays. It was found that dieckol, a major component of the extract increased the tumoricidal activity of cisplatin and even reduced the associated nephrotoxicity. This study indicates the potential of phlorotannins to be used in conjunction with tumor drugs to enhance their effect and to protect the normal cells from side effects like kidney damage.
Montero et al. (2016) employed pressurized liquid extraction for obtaining phlorotannins from Sargassum muticum collected from different locations. Structural characterization by HILIC × RP-DAD-MS/MS method revealed fuhalols, hydroxyfuhalols and phlorethols as the most abundant class of phlorotannin compounds in the extract. Anti-proliferative effect on Human colon cancer cell line HT-29 by MTT assay indicated that the cytotoxic effects of phlorotannins can be well correlated to their concentration in the extract. Further, the effect of environmental factors in the content of phlorotannins was highlighted.
Abdelhamid et al. (2019) used microwave-assisted extraction for obtaining phlorotannins from Cystoseira sedoides and evaluated its anticancer activity against human breast cancer cell line, MCF-7 by in vitro assays. Flow cytometry results showed that the phlorotannins had apoptotic activity and the effects were concentration-dependent. Further, a 3D multicellular tumor spheroid growth inhibition study indicated that phlorotannins had penetrated the tumor spheroids indicating the extracts had potential apoptotic activity.
Dutot et al. (2021) studied the chemopreventive potential of phlorotannins based on a benzopyrene induced cytotoxicity study. It was observed that phlorotannin administration helped in counteracting the cytotoxic affects induced by benzopyrene through several pathways such as by preventing the induction of cytochrome P450 activity by benzopyrene, by inhibiting the activation of P2X7 receptor whose activation is reported to induce anti-apoptotic effects, inhibiting actin rearrangement and the production of reactive oxygen species. The findings of the study highlighted the efficacy of phlorotannins as chemopreventive agents.
As a shelf life enhancer
The antimicrobial activity of phlorotannins suggest that they hold significant potential as natural preservatives in food systems. However, successful integration of phlorotannins into food systems depends on maintaining structural integrity under different storage conditions. Kang et al. (2012) extracted dieckol from E. cava using conventional solvent extraction followed by fractionation and analyzed its thermal stability. Thermal stability was assessed by storing phlorotannin compound at elevated temperatures (30, 60, and 90 °C) for 7 days and compared its radical scavenging activities using different assays by keeping ascorbic acid as the positive control. It was found that dieckol has a high degree of thermal stability up to 90 °C when compared with ascorbic acid. The cellular protective effect of dieckol against oxidative stress induced apotosis and necrosis was also confirmed using in vivo assays. The study suggested the potential of dieckol as a natural antioxidant in food systems especially where high storage temperature is employed.
Similarly, Kirke et al. (2017) reported that low molecular weight phlorotannins isolated from F. vesiculosus exhibited high antioxidant stability especially when exposed to different storage conditions signifying their potential as food preservatives. Sharifian et al. (2019) analyzed the effect of phlorotannins on melanosis and quality changes of Pacific white shrimp (Litopenaeus vannamei) during iced storage. They reported that shrimp treated with 5% phlorotannins exhibited the least melanosis score, lipid oxidation, better microbial and sensory quality suggesting its use as a melanosis inhibitor for shrimp storage.
Hyaluronidase activity
Phlorotannins are reported to possess significant hyaluronidase activity, an endopeptidase enzyme that is of great significance in the cosmetic and dermatosurgery sector. Ferreres et al (2012) reported that the purified phlorotannin extracts from brown seaweeds significantly inhibit hyaluronidase indicating that they can be used as potent cosmeceutical agent to fight skin aging. Fayad et al. (2017) studied the hyaluronidase activity of phlorotannins obtained from Padina pavonica. Out of the four different extraction techniques (Pressurized liquid extraction (PLE), microwave assisted extraction, supercritical fluid extraction and electroporation) employed, water extracts by PLE had the highest anti-hyaluronidase activity as revealed by the capillary electrophoresis assay. Arunkumar et al (2021) have also reported the anti-hyaluronidase activity of Sargassum tenerrimum extracts owing to their high phlorotannin content.
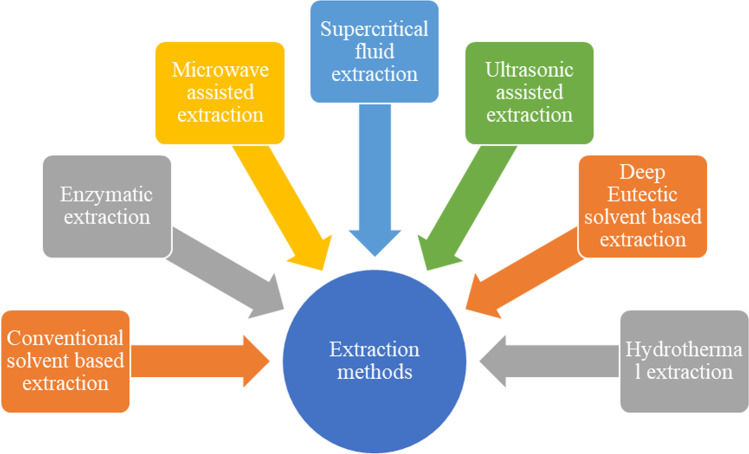
Extraction methods
Because of the potential of phlorotannins in terms of their biological activities and broader applications, their extraction has been geared up to a greater extent. Generally, solvent based extraction has been employed for obtaining seaweed polyphenols. The most commonly employed solvents for phlorotannin extraction include ethanol, acetone, methanol or their aqueous mixtures (Sathy et al. 2017; Hermund et al. 2018; Lopes et al. 2018; Zhang et al. 2018; Catarino et al. 2019). The efficiency of solvent-based extraction methods are reported to be influenced by a number of factors such as polarity of solvent, solvent to sample ratio, pH, temperature, drying method, time, pre-treatments etc. (Kim et al. 2013; Leyton et al. 2016; Li et al. 2017). Cruces et al. (2016) compared the influence of different modes of drying on the antioxidant activity of phlorotannins and reported that freeze drying was the most efficient method in retaining the antioxidant activity. Even though the conventional solvent-based extraction protocols are being used extensively, they have serious issues in terms of the environment friendliness, sustainability, solvent storage and disposal etc. Hence taking into account the disadvantages of conventional extraction protocols, several other techniques such as supercritical fluid, ultrasound microwave assisted, deep eutectic solvents, etc. are currently being explored for phlorotannin extraction (Fig. 2).
Fig. 2.
Phlorotannin Extraction methods
Solvent based conventional extraction
Heffernan et al. (2015) carried out solid liquid extraction using aqueous ethanol followed by partitioning for obtaining phlorotannins from five seaweeds—Fucus serratus, F. vesiculosus, Himanthalia elongata and Cystoseira nodicaulis. The structural diversity of phlorotannins in the extracts was elucidated by UPLC-QQQ-MS and indicated the presence of low molecular weight phlorotannins with monomers in the range of 4–12. The study also highlighted that environmental factors had a significant role for variations in phlorotannin content in different species. Kim et al. (2013) optimized the extraction of phlorotannins from Eisenia bicyclis by giving a 180 min washing time initially followed by extraction with absolute ethanol (algae to solvent ratio-1:2) for 6 h at room temperature. For determination of phlorotannins, they employed hydrophilic interaction chromatography which gave a good linearity.
Leyton et al. (2017a, b) studied the effect of different solvents and pre-treatment on the phlorotannin content in Macrocystis pyrifera and found that a pre-treatment with hexane followed by aqueous extraction can increase the yield of phlorotannins of the class of phloroeckol and a tetramer of phloroglucinol. Li et al. (2017) optimized an extraction protocol for isolation of phlorotannins from Sargassum fusiforme subjected to a conventional extraction protocol using 30% aqueous ethanol (sample to solvent ratio: 1:5). Further fractionation of the extract with ethyl acetate resulted in production of phlorotannin rich extracts with high bioactivity. Further structural characterization has revealed that most of the extracted compounds belonged to the class of fuhalols followed by phloroethols. The study has highlighted the structural diversity of phlorotannins as well as the challenges involved in its structural elucidation.
Leyton et al. (2017a, b) optimized an extraction protocol for simultaneously obtaining phlorotannin and carbohydrates from M. pyrifera by combining an enzymatic treatment using enzyme obtained from marine fungus, Alternaria sp. with hydrolysis. The optimized extraction protocol resulted in a yield of 89.67 wt %.and 2.14 wt % for carbohydrates and phlorotannins, respectively. Hermund et al. (2018) used solvent extraction using aqueous ethanol followed by fractionation for obtaining phlorotannins from F. vesiculosus. Structural elucidation of the extracts using Ultra-high performance liquid chromatography-DAD-quadrupole time of flight mass spectrometry (UHPLC-DAD-QTOFMS) indicated a molecular weight range of 374 to 870 Da. The study highlighted that the low molecular weight phlorotannins possessed superior antioxidant activities and their activities decreased with the increase in polymerization.
Lopes et al. (2018) screened for phlorotannin content in Fucus extracts using advanced mass spectrophotometric methods and reported that they are of low molecular weight. Catarino et al. (2019) optimized extraction of phlorotannins from F. vesiculosus and reported 67% acetone as an effective solvent. Characterization by UHPLC-MS indicated that the extracts were composed of fucols, fucophlorethols, fuhalols and other phlorotannin derivatives. Further enzymatic assays revealed that the extracts shown promising inhibition against enzymes such as α-glucosidase, α-amylase and pancreatic lipase indicating their potential in combating metabolic disorders such as diabetes and obesity.
Sustainable extraction technologies for isolation of phlorotannins
Greener extraction techniques are gaining momentum recently especially due to the environment friendliness of such methods. He et al. (2013) found that microwave assisted extraction protocol was more effective for isolating antiproliferative phlorotannins from Saccharina japonica than conventional solvent based method. Based on their findings, 55% ethanol worked effectively as solvent for concentrating maximum phlorotannins in an experiment duration of 25 min, temperature 60° C and microwave power 400 W.
Casas et al. (2016) employed hydrothermal extraction of alginate exhausted biomass of Sargassum muticum for obtaining phlorotannin rich extracts. The extract was subjected to adsorption and desorption studies and the desorbed product exhibited higher bioactivity than the autohydrolysed extracts. It was noted that the extract possesses significant anti-tumor and anti-proliferative activity against colon carcinoma HCT-116 cells as well as anti-inflammatory activity by inhibiting prostaglandin E2 production.
Saravana et al. (2017) studied the influence of various co-solvents such as sunflower oil, soybean oil, canola oil, ethanol, and water in enhancing the recovery of potent biomolecules such as fucoxanthin and phlorotannin S. japonica using supercritical fluid extraction. Sunflower oil and water were found to be effective solvents in extracting fucoxanthin and phlorotannin respectively. The high dielectric constant coupled with high polarity and density might have helped in swelling of the samples and thereby favoring faster permeation and extraction of polar compounds. An extraction condition with variables, temperature -48.98 °C, pressure- 300 bar, and 2.00% with water was optimized for phlorotannin extraction.
A sustainable approach for obtaining phlorotannins from Padina australis and Sargassum binderi was reported by Chia et al. (2018). Liquid biphasic system, a greener technology employing alcohol and salt mixtures was used for obtaining phlorotannins and the optimization was based on pH, sample to solvent ratio and type of alcohol. Among the different alcohols employed (methanol, ethanol, 1-propanol and 2-propanol), 2-propanol/ammonium sulphate based liquid biphasic system was found to be effective in extracting phlorotannins. About 76.1 and 91.67% phlorotannins with a purification factor of 2.49 and 1.59 were recorded from P. australis and S. binderi, respectively. Overall, the results of the study highlighted that it is a cost-effective extraction technique which can give high yield of phlorotannins and high recovery of salt solution.
Zhang et al. (2018) compared different extraction techniques such as conventional solvent, room temperature based aqueous extraction, microwave assisted and hydrothermal extraction for obtaining phlorotannins from Carpophyllum flexuosum. Among the extraction techniques employed, microwave assisted extraction (MAE) using water as solvent was found to be most effective with shorter extraction time and better purity. Cell wall degradation was reported to be high in MAE treatments as revealed by the scanning electron micrographs and might have aided in the further release of phlorotannins. Thermal stability study indicated that phlorotannins are powerful antioxidants than ascorbic acid and hence can be used as natural antioxidants. Further, the major class of extracted phlorotannins belonged to fuhalols as revealed by NMR spectroscopy.
Obluchinskaya et al. (2019) reported the extraction of phlorotannins using eutectic solvent from. F. vesiculosus and Ascophyllum nodosum. Different natural deep eutectic solvents (NADES) compositions were prepared using choline chloride, glucose, betaine as hydrogen bond acceptor and lactic acid, malic acid and glycerin as hydrogen bond donor for extracting phlorotannins. Aqueous solution based on choline chloride and lactic acid were the most effective in extracting phlorotannins. However, the extraction efficiency of the solvents depends depend to a greater extent on the water content and hence if the NADES solutions are to be diluted, it should be optimized by taking into account the extraction efficiency and polarity of the final mixture.
Dong et al. (2019) optimized a high temperature extraction protocol for obtaining phlorotannins, mostly, low molecular weight, from Undaria pinnatifida sporophyll, which is a major by-product generated during U. pinnatifida processing using 52% ethanol as solvent. Further, in vitro studies indicated that it had significant antioxidant activity and were not toxic at tested concentrations on the RAW 264.7 cells. Moreover, phlorotannins exhibited anti-inflammatory effect as evidenced by the decreased nitric acid production by downregulating the expression of inducible nitric oxide synthase.
Ummat et al. (2020) performed ultrasonic assisted extraction using aqueous ethanol for obtaining phlorotannins from brown seaweeds. The yield and antioxidant activity of phlorotannins isolated using ultrasonic assisted method were higher than that of the conventional solvent extraction method suggesting the effectiveness of former in isolating seaweed polyphenolics. Vázquez-Rodríguez et al. (2020) explored and optimized an ultrasonic assisted method for obtaining phlorotannins and polysaccharides from Silvetia compressa. an edible brown algae mostly native to Baja California coast of Mexico. The optimization was achieved by modulating extraction temperature, ultrasound power density, solvent ratio and the ethanol concentration. The findings of the study highlighted that ultrasonic assisted extraction can effectively enhance extraction of biomolecules by disrupting cell walls and thereby increasing the permeation of solvents into the cellular systems. The study further emphasized that aqueous extraction solvents are effective extraction agents as phlorotannins are highly polar in nature.
Amarante et al. (2020) optimized a microwave assisted extraction protocol for obtaining phlorotannins from F. vesiculosus using 57% aqueous ethanol as solvent yielding about 9.8 ± 1.8 mg g−1 dry wt extract. When compared with the conventional extraction, the microwave assisted extraction method was inferior in terms of the yield and its ability to scavenge free radicals. The enzymatic assays indicated that the extracts had anti-diabetic effects, effectively inhibiting the activity of α-glucosidase, a key enzyme involved in control and prevention of diabetes.
Quantification/determination of phlorotannins
Phlorotannins, if in purest form, can find many application as a bioactive ingredient in food, drugs, cosmetics etc. For any such applications, extraction followed by purification and determination of the various classes of phlorotannins is a pre-requisite. Leyton et al. (2017a, b) studied the purification of phlorotannins from M. pyrifera using different types of macroporous resin. Of the different resins employed, the highest purification was obtained with XAD-16 N exhibiting an adsorption capacity and desorption ratio of 183 mg phlorotannins g−1 resin and 38.2%, respectively. Kim et al. (2014) also report purified crude phlorotannin extracts using macroporous resins for broadening its applications.
Zhou et al. (2019) showed that size exclusion chromatography coupled with high-speed counter-current chromatography can be utilized as a successful method for obtaining purified phlorotannins from brown algae.
Determination of phlorotannin compounds is often cumbersome owing to their structural complexity. Previously, determination of phlorotannins was mostly achieved through the colorimetric assay used for determination of total phenolics, viz, Folin-Ciocalteu (FC) method (Sathy et al. 2017). With the advances in analytical sciences, chromatrophic techniques are being employed for phlorotannin determination. As a result, High pressure Liquid Chromatography (HPLC) is being widely employed for its quantitative and qualitative determination. Kim et al. (2013) used an advanced HPLC method, Hydrophilic interaction chromatography (HILIC), for determination of phlorotannins from extracts of E. bicyclis. The method was validated and was reported to give good linearity. It was further utilized for studying the seasonal variations in phlorotannin content with the highest phlorotannin content noticed in summer.
Chromatographic techniques coupled with mass spectrometry and Nuclear Magnetic Resonance (NMR) are recently being employed for the structural determination of phlorotannins (Montero et al. 2016; Hermund et al. 2018; Lopes et al. 2018). Jégou et al. (2015) employed quantitative NMR (qNMR) to quantify phlorotannins from Cystoseira tamariscifolia and compared its pros and cons with that of FC assay and chromatographic techniques. They reported that qNMR provides a reliable quantification of phlorotannins with more specificity, less sample preparation steps.
Conclusion and future prospects
Marine bioactive compounds are considered as potential candidates in biomedical/nutraceutical sectors, as safe and effective alternatives to their synthetic counter parts. Seaweed based phenolic class of compounds, phlorotannins have been explored for their bioactivities and are in high demand, especially in the cosmetic and nutraceuticals sector globally. The high degree of antioxidant and antimicrobial activity of phlorotannins suggests that they can be used as substitutes for synthetic preservatives. However, the structural diversity of phlorotannins directs the difficulties involved in mapping their content in different seaweed species. By employing advanced chromatographic/separational techniques, a complete structural elucidation of phlorotannins must be carried out as the bioactivity are highly related to their chemical structure. Though several in vivo and invitro studies have been carried out to establish their bioactivities, clinical trials should be carried out to establish its safety, bioavailability etc. Further, systematic efforts should be undertaken to develop, popularize and commercialize phlorotannin-based nutraceutical/nutraceutical supplements for human health care aspects.
Acknowledgements
The authors are grateful to Director, CIFT, Cochin for providing necessary facilities and support needed for the research.
Author contribution
Lekshmi R.G.Kumar: Conceptualization; Data curation; Writing—original draft; Writing—review & editing; Anas K.K., Tejpal C.S., Preethy Treesa Paul, Chatterjee N.S., Anupama T.K.: Writing—review & editing, Suseela Mathew, Ravishankar C.N.: Formal analysis; Investigation & Writing—review & editing.
Declarations
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdelhamid A, Lajili S, Elkaibi MA, Ben Salem Y, Abdelhamid A, Muller CD, Majdoub H, Kraiem J, Bouraoui A. Optimized extraction, preliminary characterization and evaluation of the in vitro anticancer activity of phlorotannin-rich fraction from the brown seaweed, Cystoseira sedoides. J Aquat Food Prod Technol. 2019;28:892–909. doi: 10.1080/10498850.2019.1662865. [DOI] [Google Scholar]
- Ahn G, Amagai Y, Matsuda A, Kang SM, Lee W, Jung K, Oida K, Jang H, Ishizaka S, Matsuda K, Jeon YJ, Jee Y, Matsuda H, Tanaka A. Dieckol, a phlorotannin of Ecklonia cava, suppresses IgE-mediated mast cell activation and passive cutaneous anaphylactic reaction. Exp Dermatol. 2015;24:968–970. doi: 10.1111/exd.12814. [DOI] [PubMed] [Google Scholar]
- Amarante SJ, Catarino MD, Marçal C, Silva A, Ferreira R, Cardoso SM. Microwave-assisted extraction of phlorotannins from Fucus vesiculosus. Mar Drugs. 2020;18:559. doi: 10.3390/md18110559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunkumar K, Raj R, Raja R, Carvalho IS. Brown seaweeds as a source of anti-hyaluronidase compounds. S Afr J Bot. 2021;139:470–477. doi: 10.1016/j.sajb.2021.03.036. [DOI] [Google Scholar]
- Balboa EM, Cond E, Moure A, Falqué E, Domínguez H (2013) In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem 138:1764–1785 [DOI] [PubMed]
- Bai R, Yao C, Zhong Z, Ge J, Bai Z, Ye X, Xie T, Xie Y. Discovery of natural anti-inflammatory alkaloids: potential leads for the drug discovery for the treatment of inflammation. Eur J Med Chem. 2021;213:113165. doi: 10.1016/j.ejmech.2021.113165. [DOI] [PubMed] [Google Scholar]
- Bai Y, Chen X, Qi H. Characterization and bioactivity of phlorotannin loaded protein-polysaccharide nanocomplexes. LWT. 2022;155:112998. doi: 10.1016/j.lwt.2021.112998. [DOI] [Google Scholar]
- Barbosa M, Lopes G, Andrade PB, Valentão P. Bioprospecting of brown seaweeds for biotechnological applications: Phlorotannin actions in inflammation and allergy network. Trends Food Sci Technol. 2019;86:153–171. doi: 10.1016/j.tifs.2019.02.037. [DOI] [Google Scholar]
- Barbosa M, Lopes G, Ferreres F, Andrade PB, Pereira DM, Gil-Izquierdo Á, Valentão P. Phlorotannin extracts from Fucales: Marine polyphenols as bioregulators engaged in inflammation-related mediators and enzymes. Algal Res. 2017;28:1–8. doi: 10.1016/j.algal.2017.09.009. [DOI] [Google Scholar]
- Barbosa M, Valentão P, Ferreres F, Gil-Izquierdo Á, Andrade PB. In vitro multifunctionality of phlorotannin extracts from edible Fucus species on targets underpinning neurodegeneration. Food Chem. 2020;333:127456. doi: 10.1016/j.foodchem.2020.127456. [DOI] [PubMed] [Google Scholar]
- Casas MP, Rodríguez-Hermida V, Pérez-Larrán P, Conde E, Liveri MT, Ribeiro D, Domínguez H. In vitro bioactive properties of phlorotannins recovered from hydrothermal treatment of Sargassum muticum. Sep Purif Technol. 2016;167:117–126. doi: 10.1016/j.seppur.2016.05.003. [DOI] [Google Scholar]
- Catarino MD, Marçal C, Bonifácio-Lopes T, Campos D, Mateus N, Silva A, Pintado MM, Cardoso SM. Impact of phlorotannin extracts from Fucus vesiculosus on human gut microbiota. Mar Drugs. 2021;19:375. doi: 10.3390/md19070375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catarino MD, Silva A, Mateus N, Cardoso SM. Optimization of phlorotannins extraction from Fucus vesiculosus and evaluation of their potential to prevent metabolic disorders. Mar Drugs. 2019;17:162. doi: 10.3390/md17030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia SR, Show PL, Phang SM, Ling TC, Ong HC. Sustainable approach in phlorotannin recovery from macroalgae. J Biosci Bioeng. 2018;126:220–225. doi: 10.1016/j.jbiosc.2018.02.015. [DOI] [PubMed] [Google Scholar]
- Cho HM, Doan TP, Ha TKQ, Kim HW, Lee BW, Pham HTT, Cho TO, Oh WK. Dereplication by high-performance liquid chromatography (HPLC) with quadrupole-time-of-flight mass spectroscopy (qTOF-MS) and antiviral activities of phlorotannins from Ecklonia cava. Mar Drugs. 2019;17:149. doi: 10.3390/md17030149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creis E, Delage L, Charton S, Goulitquer S, Leblanc C, Potin P, Gall EA. Constitutive or inducible protective mechanisms against UV-B radiation in the brown alga Fucus vesiculosus? A study of gene expression and phlorotannin content responses. PLoS One. 2015;10:e0128003. doi: 10.1371/journal.pone.0128003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruces E, Rojas-Lillo Y, Ramirez-Kushel E, Atala E, López-Alarcón C, Lissi E, Gómez I. Comparison of different techniques for the preservation and extraction of phlorotannins in the kelp Lessonia spicata (Phaeophyceae): assays of DPPH, ORAC-PGR, and ORAC-FL as testing methods. J Appl Phycol. 2016;28:573–580. doi: 10.1007/s10811-015-0602-9. [DOI] [Google Scholar]
- Cui H, Yang X, Abdel-Samie MA, Lin L. Cold plasma treated phlorotannin/Momordica charantia polysaccharide nanofiber for active food packaging. Carbohydr Polym. 2020;239:116214. doi: 10.1016/j.carbpol.2020.116214. [DOI] [PubMed] [Google Scholar]
- Dong X, Bai Y, Xu Z, Shi Y, Sun Y, Janaswamy S, Yu C, Qi H. Phlorotannins from Undaria pinnatifida sporophyll: Extraction, antioxidant, and anti-inflammatory activities. Mar Drugs. 2019;17:434. doi: 10.3390/md17080434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutot M, Olivier E, Fouyet S, Magny R, Hammad K, Roulland E, Rat P, Fagon R. In Vitro Chemopreventive potential of phlorotannins-rich extract from brown algae by inhibition of benzo[a]pyrene-induced P2X7 activation and toxic effects. Mar Drugs. 2021;19:34. doi: 10.3390/md19010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom SH, Kim DH, Lee SH, Yoon NY, Kim JH, Kim TH, Chung YH, Kim SB, Kim YM, Kim HW, Kim YM. In vitro antibacterial activity and synergistic antibiotic effects of phlorotannins isolated from Eisenia bicyclis against methicillin-resistant Staphylococcus aureus. Phytother Res. 2013;27:1260–1264. doi: 10.1002/ptr.4851. [DOI] [PubMed] [Google Scholar]
- Fayad S, Nehmé R, Tannoury M, Lesellier E, Pichon C, Morin P. Macroalga Padina pavonica water extracts obtained by pressurized liquid extraction and microwave-assisted extraction inhibit hyaluronidase activity as shown by capillary electrophoresis. J Chromatogr A. 2017;1497:19–27. doi: 10.1016/j.chroma.2017.03.033. [DOI] [PubMed] [Google Scholar]
- Ferdouse F, Holdt SL, Smith R, Murúa P, Yang Z (2018) The global status of seaweed production, trade and utilization. Globefish Research Programme, Vol. 124, FAO, Rome
- Ferreira CA, Januário AP, Félix R, Alves N, Lemos MF, Dias JR. Multifunctional gelatin/chitosan electrospun wound dressing dopped with Undaria pinnatifida phlorotannin-enriched extract for skin regeneration. Pharmaceutics. 2021;13:2152. doi: 10.3390/pharmaceutics13122152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreres F, Lopes G, Gil-Izquierdo A, Andrade PB, Sousa C, Mouga T, Valentão P. Phlorotannin extracts from Fucales characterized by HPLC-DAD-ESI-MSn: approaches to hyaluronidase inhibitory capacity and antioxidant properties. Mar Drugs. 2012;10:2766–2781. doi: 10.3390/md10122766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleurence J, Levine I, editors. Seaweed in health and disease prevention. Amsterdam: Elsevier; 2016. [Google Scholar]
- Gheda S, Naby MA, Mohamed T, Pereira L, Khamis A (2021) Antidiabetic and antioxidant activity of phlorotannins extracted from the brown seaweed Cystoseira compressa in streptozotocin-induced diabetic rats. Environ Sci Pollut 28:22886–22901 [DOI] [PubMed]
- Guiry MD (2014) The seaweed site: information on marine algae. Seaweed ie
- He Z, Chen Y, Chen Y, Liu H, Yuan G, Fan Y, Chen K. Optimization of the microwave-assisted extraction of phlorotannins from Saccharina japonica Aresch and evaluation of the inhibitory effects of phlorotannin-containing extracts on HepG2 cancer cells. Chin J Oceanol Limnol. 2013;31:1045–1054. doi: 10.1007/s00343-013-2321-x. [DOI] [Google Scholar]
- Heffernan N, Brunton NP, FitzGerald RJ, Smyth TJ. Profiling of the molecular weight and structural isomer abundance of macroalgae-derived phlorotannins. Mar Drugs. 2015;13:509–528. doi: 10.3390/md13010509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermund DB, Plaza M, Turner C, Jonsdottir R, Kristinsson HG, Jacobsen C, Nielsen KF. Structure dependent antioxidant capacity of phlorotannins from Icelandic Fucus vesiculosus by UHPLC-DAD-ECD-QTOFMS. Food Chem. 2018;240:904–909. doi: 10.1016/j.foodchem.2017.08.032. [DOI] [PubMed] [Google Scholar]
- Hwang J, Yang HW, Lu YA, Je JG, Lee HG, Fernando KH, Jeon YJ, Ryu B. Phloroglucinol and dieckol isolated from Ecklonia cava suppress impaired diabetic angiogenesis; A study of in-vitro and in-vivo. Biomed Pharmacother. 2021;1:111431. doi: 10.1016/j.biopha.2021.111431. [DOI] [PubMed] [Google Scholar]
- Imbs TI, Silchenko AS, Fedoreev SA, Isakov VV, Ermakova SP, Zvyagintseva TN. Fucoidanase inhibitory activity of phlorotannins from brown algae. Algal Res. 2018;32:54–59. doi: 10.1016/j.algal.2018.03.009. [DOI] [Google Scholar]
- Jégou C, Kervarec N, Cérantol S, Bihannic I, Stiger-Pouvreau V. NMR use to quantify phlorotannins: The case of Cystoseira tamariscifolia, a phloroglucinol-producing brown macroalga in Brittany (France) Talanta. 2015;135:1–6. doi: 10.1016/j.talanta.2014.11.059. [DOI] [PubMed] [Google Scholar]
- Jung HA, Jin SE, Ahn BR, Lee CM, Choi JS. Anti-inflammatory activity of edible brown alga Eisenia bicyclis and its constituents fucosterol and phlorotannins in LPS-stimulated RAW264. 7 macrophages. Food Chem Toxicol. 2013;59:199–206. doi: 10.1016/j.fct.2013.05.061. [DOI] [PubMed] [Google Scholar]
- Kang MC, Cha SH, Wijesinghe WAJP, Kang SM, Lee SH, Kim EA, Song CB, Jeon YJ. Protective effect of marine algae phlorotannins against AAPH-induced oxidative stress in zebrafish embryo. Food Chem. 2013;138:950–955. doi: 10.1016/j.foodchem.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Kang MC, Kim EA, Kang SM, Wijesinghe WAJP, Yang X, Kang NL, Jeon YJ. Thermostability of a marine polyphenolic antioxidant dieckol, derived from the brown seaweed Ecklonia cava. Algae. 2012;27:205–213. doi: 10.4490/algae.2012.27.3.205. [DOI] [Google Scholar]
- Kannan RR, Aderogba MA, Ndhlala AR, Stirk WA, Van Staden J. Acetylcholinesterase inhibitory activity of phlorotannins isolated from the brown alga, Ecklonia maxima (Osbeck) Papenfuss. Food Res Int. 2013;54:1250–1254. doi: 10.1016/j.foodres.2012.11.017. [DOI] [Google Scholar]
- Kellogg J, Grace MH, Lila MA. Phlorotannins from Alaskan seaweed inhibit carbolytic enzyme activity. Mar Drugs. 2014;12:5277–5294. doi: 10.3390/md12105277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Dasagrandhi C, Kim SH, Kim BG, Eom SH, Kim YM. In vitro antibacterial activity of phlorotannins from edible brown algae, Eisenia bicyclis against streptomycin-resistant Listeria monocytogenes. Indian J Microbiol. 2018;58:105–108. doi: 10.1007/s12088-017-0693-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Yoon M, Yang H, Jo J, Han D, Jeon YJ, Cho S. Enrichment and purification of marine polyphenol phlorotannins using macroporous adsorption resins. Food Chem. 2014;162:135–142. doi: 10.1016/j.foodchem.2014.04.035. [DOI] [PubMed] [Google Scholar]
- Kim SM, Kang SW, Jeon JS, Jung YJ, Kim WR, Kim CY, Um BH. Determination of major phlorotannins in Eisenia bicyclis using hydrophilic interaction chromatography: Seasonal variation and extraction characteristics. Food Chem. 2013;138:2399–2406. doi: 10.1016/j.foodchem.2012.11.057. [DOI] [PubMed] [Google Scholar]
- Kim TH, Ku SK, Lee T, Bae JS. Vascular barrier protective effects of phlorotannins on HMGB1-mediated proinflammatory responses in vitro and in vivo. Food Chem Toxicol. 2012;50:2188–2195. doi: 10.1016/j.fct.2012.03.082. [DOI] [PubMed] [Google Scholar]
- Kirke DA, Rai DK, Smyth TJ, Stengel DB. An assessment of temporal variation in the low molecular weight phlorotannin profiles in four intertidal brown macroalgae. Algal Res. 2019;41:101550. doi: 10.1016/j.algal.2019.101550. [DOI] [Google Scholar]
- Kirke DA, Smyth TJ, Rai DK, Kenny O, Stengel DB. The chemical and antioxidant stability of isolated low molecular weight phlorotannins. Food Chem. 2017;221:1104–1112. doi: 10.1016/j.foodchem.2016.11.050. [DOI] [PubMed] [Google Scholar]
- Kwon HJ, Ryu YB, Kim YM, Song N, Kim CY, Rho MC, Jeong JH, Cho KO, Lee WS, Park SJ. In vitro antiviral activity of phlorotannins isolated from Ecklonia cava against porcine epidemic diarrhea coronavirus infection and hemagglutination. Bioorg Med Chem. 2013;21:4706–4713. doi: 10.1016/j.bmc.2013.04.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le QT, Li Y, Qian ZJ, Kim MM, Kim SK. Inhibitory effects of polyphenols isolated from marine alga Ecklonia cava on histamine release. Process Biochem. 2009;44:168–176. doi: 10.1016/j.procbio.2008.10.002. [DOI] [Google Scholar]
- Lee S, Youn K, Kim DH, Ahn MR, Yoon E, Kim OY, Jun M. Anti-neuroinflammatory property of phlorotannins from Ecklonia cava on Aβ25-35-induced damage in PC12 cells. Mar Drugs. 2019;17:7. doi: 10.3390/md17010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Ko SC, Kang MC, Lee DH, Jeon YJ. Octaphlorethol A, a marine algae product, exhibits antidiabetic effects in type 2 diabetic mice by activating AMP-activated protein kinase and upregulating the expression of glucose transporter 4. Food Chem Toxicol. 2016;91:58–64. doi: 10.1016/j.fct.2016.02.022. [DOI] [PubMed] [Google Scholar]
- Leyton A, Pezoa-Conte R, Barriga A, Buschmann AH, Mäki-Arvela P, Mikkola JP, Lienqueo ME. Identification and efficient extraction method of phlorotannins from the brown seaweed Macrocystis pyrifera using an orthogonal experimental design. Algal Res. 2016;16:201–208. doi: 10.1016/j.algal.2016.03.019. [DOI] [Google Scholar]
- Leyton A, Pezoa-Conte R, Mäki-Arvela P, Mikkola JP, Lienqueo ME. Improvement in carbohydrate and phlorotannin extraction from Macrocystis pyrifera using carbohydrate active enzyme from marine Alternaria sp. as pretreatment. J Appl Phycol. 2017;29:2039–2048. doi: 10.1007/s10811-017-1141-3. [DOI] [Google Scholar]
- Leyton A, Vergara-Salinas JR, Pérez-Correa JR, Lienqueo ME. Purification of phlorotannins from Macrocystis pyrifera using macroporous resins. Food Chem. 2017;237:312–319. doi: 10.1016/j.foodchem.2017.05.114. [DOI] [PubMed] [Google Scholar]
- Li Y, Fu X, Duan D, Liu X, Xu J, Gao X. Extraction and identification of phlorotannins from the brown alga, Sargassum fusiforme (Harvey) Setchell. Mar Drugs. 2017;15:49. doi: 10.3390/md15020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Lee SH, Le QT, Kim MM, Kim SK. Anti-allergic effects of phlorotannins on histamine release via binding inhibition between IgE and FcεRI. J Agri Food Chem. 2008;56:12073–12080. doi: 10.1021/jf802732n. [DOI] [PubMed] [Google Scholar]
- Lopes G, Barbosa M, Andrade PB, Valentão P. Phlorotannins from Fucales: Potential to control hyperglycemia and diabetes-related vascular complications. J Appl Phycol. 2019;31:3143–3152. doi: 10.1007/s10811-019-01816-7. [DOI] [Google Scholar]
- Lopes G, Barbosa M, Vallejo F, Gil-Izquierdo Á, Andrade PB, Valentão P, Pereira DM, Ferreres F. Profiling phlorotannins from Fucus spp. of the Northern Portuguese coastline: Chemical approach by HPLC-DAD-ESI/MSn and UPLC-ESI-QTOF/MS. Algal Res. 2018;29:113–120. doi: 10.1016/j.algal.2017.11.025. [DOI] [Google Scholar]
- Lopes G, Pinto E, Andrade PB, Valentão P. Antifungal activity of phlorotannins against dermatophytes and yeasts: approaches to the mechanism of action and influence on Candida albicans virulence factor. PLoS One. 2013;8:e72203. doi: 10.1371/journal.pone.0072203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Ito C, Itoigawa M, Shibata T. Three phlorotannins from Sargassum carpophyllum are effective against the secretion of allergic mediators from antigen-stimulated rat basophilic leukemia cells. Food Chem. 2022;3:131992. doi: 10.1016/j.foodchem.2021.131992. [DOI] [PubMed] [Google Scholar]
- Montero L, Sánchez-Camargo AP, García-Cañas V, Tanniou A, Stiger-Pouvreau V, Russo M, Rastrelli L, Cifuentes A, Ibáñez HM, E, Anti-proliferative activity and chemical characterization by comprehensive two-dimensional liquid chromatography coupled to mass spectrometry of phlorotannins from the brown macroalga Sargassum muticum collected on North-Atlantic coasts. J Chromatogr A. 2016;1428:115–125. doi: 10.1016/j.chroma.2015.07.053. [DOI] [PubMed] [Google Scholar]
- Nair D, Vanuopadath M, Balasubramanian A, Iyer A, Ganesh S, Anil AN, Vikraman V, Pillai P, Nair BC, Pai BG, JG, Nair SS, Phlorotannins from Padina tetrastromatica: structural characterisation and functional studies. J Appl Phycol. 2019;31:3131–3141. doi: 10.1007/s10811-019-01792-y. [DOI] [Google Scholar]
- Obluchinskaya ED, Daurtseva AV, Pozharitskaya ON, Flisyuk EV, Shikov AN. Natural Deep Eutectic Solvents as alternatives for extracting phlorotannins from brown algae. Pharm Chem J. 2019;53:243–247. doi: 10.1007/s11094-019-01987-0. [DOI] [Google Scholar]
- Park JY, Kim JH, Kwon JM, Kwon HJ, Jeong HJ, Kim YM, Kim D, Lee WS, Ryu YB. Dieckol, a SARS-CoV 3CLpro inhibitor, isolated from the edible brown algae Ecklonia cava. Bioorg Med Chem. 2013;21:3730. doi: 10.1016/j.bmc.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SR, Kim JH, Jang HD, Yang SY, Kim YH. Inhibitory activity of minor phlorotannins from Ecklonia cava on α-glucosidase. Food Chem. 2018;257:128–134. doi: 10.1016/j.foodchem.2018.03.013. [DOI] [PubMed] [Google Scholar]
- Saravana PS, Getachew AT, Cho YJ, Choi JH, Park YB, Woo HC, Chun BS. Influence of co-solvents on fucoxanthin and phlorotannin recovery from brown seaweed using supercritical CO2. J Supercrit Fluids. 2017;120:295–303. doi: 10.1016/j.supflu.2016.05.037. [DOI] [Google Scholar]
- Sathy R, Kanaga N, Sankar P, Jeeva S. Antioxidant properties of phlorotannins from brown seaweed Cystoseira trinodis (Forsskål) C. Agardh Arab J Chem. 2017;10:S2608–S2614. doi: 10.1016/j.arabjc.2013.09.039. [DOI] [Google Scholar]
- Sharifian S, Shabanpour B, Taheri A, Kordjazi M. Effect of phlorotannins on melanosis and quality changes of Pacific white shrimp (Litopenaeus vannamei) during iced storage. Food Chem. 2019;298:124980. doi: 10.1016/j.foodchem.2019.124980. [DOI] [PubMed] [Google Scholar]
- Shrestha S, Johnston MR, Zhang W, Smid SD. A phlorotannin isolated from Ecklonia radiata, dibenzodioxin-fucodiphloroethol, inhibits neurotoxicity and aggregation of β-amyloid. Phytomed Plus. 2021;1:100125. doi: 10.1016/j.phyplu.2021.100125. [DOI] [Google Scholar]
- Son M, Oh S, Choi J, Jang JT, Choi CH, Park KY, Son KH, Byun K (2020) The phlorotannin-rich fraction of Ecklonia cava extract attenuated the expressions of the markers related with inflammation and leptin resistance in adipose tissue. Int J Endocrinol 2020. Article ID 9142134 [DOI] [PMC free article] [PubMed]
- Sugiura Y, Tanaka R, Katsuzaki H, Imai K, Matsushita T. The anti-inflammatory effects of phlorotannins from Eisenia arborea on mouse ear edema by inflammatory inducers. J Funct Foods. 2013;5:2019–2023. doi: 10.1016/j.jff.2013.08.010. [DOI] [Google Scholar]
- Surendhiran D, Cui H, Lin L. Encapsulation of phlorotannin in alginate/PEO blended nanofibers to preserve chicken meat from Salmonella contaminations. Food Packag Shelf Life. 2019;21:100346. doi: 10.1016/j.fpsl.2019.100346. [DOI] [Google Scholar]
- Tang J, Wang W, Chu W. Antimicrobial and anti-quorum sensing activities of phlorotannins from seaweed (Hizikia fusiforme) Front Cell Infect Microbiol. 2020;10:652. doi: 10.3389/fcimb.2020.586750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ummat V, Tiwari BK, Jaiswal AK, Condon K, Garcia-Vaquer M, O’Doherty J, O’Donnell RG. Optimisation of ultrasound frequency, extraction time and solvent for the recovery of polyphenols, phlorotannins and associated antioxidant activity from brown seaweeds. Mar Drugs. 2020;18:250. doi: 10.3390/md18050250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Rodríguez B, Gutiérrez-Uribe JA, Antunes-Ricardo M, Santos-Zea, L, Cruz-Suárez LE (2020) Ultrasound-assisted extraction of phlorotannins and polysaccharides from Silvetia compressa (Phaeophyceae). J Appl Phycol 32:1441–1453
- Vázquez-Rodríguez B, Santos-Zea L, Heredia-Olea E, Acevedo-Pacheco L, Santacruz A, Gutiérrez-Uribe JA, Cruz-Suárez LE. Effects of phlorotannin and polysaccharide fractions of brown seaweed Silvetia compressa on human gut microbiota composition using an in vitro colonic model. J Funct Foods. 2021;1:104596. doi: 10.1016/j.jff.2021.104596. [DOI] [Google Scholar]
- Venkatesan J Keekan KK, Anil S, Bhatnagar I, Kim SK (2019) Phlorotannins. Encyclopedia of Food Chemistry 2019:515–527
- Wang C, Jiang D, Sun Y, Gu Y, Ming Y, Zheng J, Yu C, Chen X, Qi H. Synergistic effects of UVA irradiation and phlorotannin extracts of Laminaria japonica on properties of grass carp myofibrillar protein gel. J Sci Food Agric. 2021;101:2659–2667. doi: 10.1002/jsfa.10890. [DOI] [PubMed] [Google Scholar]
- Wijesinghe WAJP, Ahn G, Lee WW, Kang MC, Kim EA, Jeon YJ. Anti-inflammatory activity of phlorotannin-rich fermented Ecklonia cava processing by-product extract in lipopolysaccharide-stimulated RAW 264.7 macrophages. J Appl Phycol. 2013;25:1207–1213. doi: 10.1007/s10811-012-9939-5. [DOI] [Google Scholar]
- Yang HK, Jung MH, Avunje S, Nikapitiya C, Kang SY, Ryu YB, Lee WS, Jung SJ. Efficacy of algal Ecklonia cava extract against viral hemorrhagic septicemia virus (VHSV) Fish Shellfish Immunol. 2018;72:273–281. doi: 10.1016/j.fsi.2017.10.044. [DOI] [PubMed] [Google Scholar]
- Yang YI, Ahn JH, Choi YS, Choi JH. Brown algae phlorotannins enhance the tumoricidal effect of cisplatin and ameliorate cisplatin nephrotoxicity. Gynecol Oncol. 2015;136:355–364. doi: 10.1016/j.ygyno.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Yu S, Duan M, Sun J, Jiang H, Zhao J, Tong C, Pang J, Wu C (2022) Immobilization of phlorotannins on nanochitin: A novel biopreservative for refrigerated sea bass (Lateolabrax japonicus) fillets. Int J Biol Macromol 626-634 [DOI] [PubMed]
- Zhang R, Yuen AK, Magnusson M, Wright JT, de Nys R, Masters AF, Maschmeyer T. A comparative assessment of the activity and structure of phlorotannins from the brown seaweed Carpophyllum flexuosum. Algal Res. 2018;29:130–141. doi: 10.1016/j.algal.2017.11.027. [DOI] [Google Scholar]
- Zhou X, Yi M, Ding L, He S, Yan X. Isolation and purification of a neuroprotective phlorotannin from the marine algae Ecklonia maxima by size exclusion and high-speed counter-current chromatography. Mar Drugs. 2019;17:212. doi: 10.3390/md17040212. [DOI] [PMC free article] [PubMed] [Google Scholar]