Abstract
Introduction:
De-resuscitation practices in septic patients with heart failure (HF) are not well characterized. This study aimed to determine if diuretic initiation within 48 hours of intensive care unit (ICU) admission was associated with a positive fluid balance and patient outcomes.
Methods:
This single-center, retrospective cohort study included adult patients with an established diagnosis of HF admitted to the ICU with sepsis or septic shock. The primary outcome was the incidence of positive fluid balance in patients receiving early (<48 hours) versus late (>48 hours) initiation of diuresis. Secondary outcomes included hospital mortality, ventilator-free days, and hospital and ICU length of stay. Continuous variables were assessed using independent t-test or Mann-Whitney U, while categorical variables were evaluated using the Pearson Chi-squared test.
Results:
A total of 101 patients were included. Positive fluid balance was significantly reduced at 72 hours (−139 mL vs 4370 mL, P < .001). The duration of mechanical ventilation (4 vs 5 days, P = .129), ventilator-free days (22 vs 18.5 days, P = .129), and in-hospital mortality (28 (38%) vs 12 (43%), P = .821) were similar between groups. In a subgroup analysis excluding patients not receiving renal replacement therap (RRT) (n = 76), early diuretics was associated with lower incidence of mechanical ventilation (41 [73.2%] vs 20 (100%), P = .01) and reduced duration of mechanical ventilation (4 vs 8 days, P = .018).
Conclusions:
Diuretic use within 48 hours of ICU admission in septic patients with HF resulted in less incidence of positive fluid balance. Early diuresis in this unique patient population warrants further investigation.
Keywords: Sepsis, fluid stewardship, heart failure, diuretics, critical care
Introduction
De-resuscitation in sepsis with heart failure (HF) lacks data to guide practice.1,2 Aggressive fluid resuscitation forms the foundation of early sepsis management, yet optimal fluid volumes and the timing of their removal (eg, diuresis) remain unestablished. Further, guidelines make no recommendations for fluid management in septic patients with HF beyond initial resuscitation practices.1-3 Given the concerns for fluid overload in severe HF patients, the interaction between sepsis and comorbid HF on fluid management in the de-resuscitation phase of sepsis warrants evaluation.
In sepsis, fluid replenishes the extravasated intravascular volume from extensive vasodilation and increases cardiac output by augmenting preload. With co-morbid HF, the heart may fail to respond adequately to fluids due to myocardial overstretching from chronically expanded intravascular volume common to HF. 4 Increased intravascular volume in HF patients may also precipitate pulmonary edema requiring mechanical ventilation. 5
Several studies support guideline directed fluid resuscitation (30 mL/kg of crystalloid by 3 hours) in HF showing either neutral or improved outcomes with traditional fluid boluses in sepsis and septic shock with comorbid HF.6-13 However, the timing and degree of de-resuscitation after initial fluids is essential as fluid overload and positive fluid balances have been associated with poor outcomes in septic and intensive care patients.14-17
The ROSE curve provides a useful model of fluid stewardship in the septic patient with four specific phases: initial fluid Resuscitation, Optimization of hemodynamics, Stabilization, and Evacuation of fluid after stability is achieved.18,19 Most research has examined the resuscitation phase creating a knowledge gap regarding the optimal de-resuscitation (evacuation) timeline, especially in unique populations like HF. 20 Early fluid evacuation strategies with diuretics have demonstrated improved outcomes.21,22 Recently, Dhondup et al. found an association between both a net negative 1 L daily fluid balance and cumulative net negative fluid balance with lower mortality and ICU length of stay. 23 These de-resuscitation considerations have additive importance in HF patients, as positive hospital weight gain led to higher rates of readmission and reduced survival. 24 Indeed, most hospitalized patients with severe HF (left ventricular ejection fraction [LVEF] < 40%) rely on high doses of loop diuretics to achieve decongestion, 25 and loop diuretic use may lead to lower mortality in critically ill patients with a positive fluid balance (eg, those after fluid resuscitation in sepsis). 26
Taken together, both aggressive fluid resuscitation followed by evacuation is likely necessary to optimize outcomes where the need for hemodynamic stabilization is quickly followed by the need for neutral and/or negative fluid balance. Because of their volume-sensitive physiology, patients with HF and reduced LVEF may be particularly harmed by fluid overload following resuscitation. 27
The purpose of this study was to determine if diuretic initiation before or after 48 hours of ICU admission reduced fluid balance and improved outcomes in ICU patients with sepsis or septic shock and comorbid HF. We hypothesized that patients who received diuretics early in their ICU course would have better outcomes (eg, reduced mechanical ventilation requirement) than those with diuretics started later in their hospital stay.
Methods
This single center, retrospective cohort study included patients if they were at least 18 years of age with an established diagnosis of heart failure (HF) and admitted to the medical or cardiac intensive care unit (ICU) with a preliminary diagnosis of sepsis or septic shock. HF was defined by documented past medical history. Patients with both preserved and reduced LVEF were included where reduced LVEF was defined as EF <40%. Sepsis and septic shock were defined by initial diagnosis codes included in the ICU admission notes. The study period included patients from October 1, 2018 to January 1, 2020. Patients were excluded if they received maintenance diuretics equivalent to their home dose or expired less than 48 hours after ICU admission.
The primary outcome was the fluid balance at 72-hours in patients receiving early (<48 hours) versus late (>48 hours) initiation of diuresis. Secondary outcomes included hospital mortality, ventilator-free days, hospital and ICU length of stay, incidence of diuretic-associated side effects including acute kidney injury (AKI) and electrolyte abnormalities, need for renal replacement therapy (RRT), and fluid balance at 24 and 48 hours after ICU admission in each group. Ventilator- free days were defined as days out of 28 that the patient was alive and free of mechanical ventilation. Patients who expired prior to 28 days were considered to have zero mechanically ventilator free days.
Data were collected through retrospective chart review of the electronic medical record. Data included demographics, sequential organ failure assessment (SOFA) score, fluid balance during the first 24, 48, and 72 hours, cumulative dose of diuretics, vasopressors, and inotropes during ICU stay, incidence of acute kidney injury (AKI), electrolyte derangements, duration of mechanical ventilation, and length of ICU and hospital stay. Fluid balance during the first 24, 48, and 72 hours was calculated from intake and output data in the medical record. Diuretic use was defined as the use of any loop diuretic within 72 hours of ICU admission. Cumulative diuretic and vasopressor doses were converted to IV furosemide (mg) and norepinephrine (mcg/kg) equivalents respectively for standardization. AKI was defined as a 1.5-fold increase from admission serum creatinine. 28 Electrolyte derangements included hyponatremia (sodium < 135 mEq/L), hypokalemia (potassium < 3.5 mEq/L), and hypocalcemia (calcium < 8.8 mg/dL). Institutional review board (IRB) approval was obtained prior to initiation of the study.
Statistical Analysis
Statistical analyses were completed using IBM SPSS Statistics for Windows, Version 25.0 (IBM Corp, Armonk, New York). Statistical significance was assessed using an alpha level of 0.05. Continuous variables were assessed using independent t-test (parametric) or Mann-Whitney U (non-parametric) and expressed as mean (standard deviation) or median (interquartile range (IQR)) respectively. Categorical variables were assessed using the Pearson Chi-squared test and results are expressed as n (%). Descriptive statistics were used on all other variables. For the primary analysis, patients were divided into two groups: early versus late diuretic use. Early diuretic use was defined as diuretic initiated within 48 hours of ICU admission; late diuretic use was defined as diuretic initiation after 48 hours of ICU admission. A post-hoc analysis was performed on the subgroup of patients not requiring RRT during admission.
Results
A total of 101 patients were included. The mean age was 63.5 (standard deviation [SD] 13.9) with 56 (55.5%) males and 54 (53.5%) having reduced LVEF. Except for median SOFA score (8.8 vs 10.9, P = .029), patient characteristics were similar between early and late diuretic groups. A total 25 patients required RRT during their admission, 17 (23.2%) in the early diuretic group and 8 (28.6%) in the late group. Full patient characteristics can be found in Table 1.
Table 1.
Patient characteristics.
| All patients (n = 101) | ⩽48 h (n = 73) | >48 h (n = 28) | P-value | |
|---|---|---|---|---|
| Age, mean (SD) | 63.52 (13.9) | 64.1 (14.6) | 61.4 (11.5) | .579 |
| Male | 56 (55.5) | 39 (53.4) | 17 (60.7) | .509 |
| Weight (kg) | 85.2 (26.1) | 88.3 (27.2) | 77.8 (21) | .065 |
| LVEF < 40% | 54 (53.5) | 41 (56.2) | 13 (46.4) | .380 |
| SIRS criteria met on admission, | 83 (82.17) | 60 (82.2) | 23 (82.1) | .995 |
| SOFA score | 9.4 (9) | 8.76 (4.26) | 10.9 (4.23) | .029 |
| Culture positiv | 53 (52.47) | 37 (50.7) | 17 (60.7) | .366 |
| Renal replacement therapy | ||||
| Total | 25 (100) | 17 (100) | 8 (100) | – |
| CRRT | 17 (68) | 10 (58.8) | 7 (87.5) | – |
| IHD | 6 (24) | 6 (35.3) | 0 (0) | – |
| PD | 2 (8) | 1 (5.9) | 1 (12.5) | – |
| MV within 24 h of ICU admission | 63 (63) | 46 (63) | 17 (61) | – |
| Source of Infection, n (%) | ||||
| Respiratory | 43 (42.57) | 35 (48.0) | 10 (35.7) | – |
| Urinary | 17 (16.83) | 10 (13.7) | 5 (17.9) | – |
| Skin/Soft tissue | 2 (1.98) | 3 (4.2) | 1 (3.6) | – |
| Unknown/Other | 40 (39.6) | 25 (34.2) | 12 (42.8) | – |
| Loop diuretic, n (%) | ||||
| Bumetanide | 8 (7.92) | 8 (11) | 0 (0) | – |
| Furosemide | 93 (92.07) | 65 (89) | 28 (100) | – |
| Chronic loop diuretic use, n (%) | 55 (54.45) | 43 (58.9) | 12 (42.9) | 0.094 |
Data are presented as n (%) or mean (SD) unless otherwise noted.
Abbreviations: LVEF, left-ventricular ejection fraction; SIRS, systemic inflammatory response syndrome; SOFA, sequential organ failure assessment; CRRT, continuous renal replacement therapy; IHD, intermittent hemodialysis; PD, peritoneal dialysis; MV, Mechanical Ventilation; ICU, Intensive care unit.
Net fluid balance was significantly less in those patients who received diuretics within 48 hours of admission at all time points (Table 2). Despite administration of significantly higher cumulative diuretic doses within the early diuretic group (320 mg vs 140 mg, P = .034), the incidence of electrolyte derangements was the same. No significant difference was observed in intubation between the early and late diuretic group (72% vs 75%, P = .856). The duration of mechanical ventilation (4 vs 5 days, P = .129) and ventilator-free days (22 vs 18.5 days, P = .129) were also similar between groups. In- hospital ortality between patient groups was also similar (28 [38%] vs 12 (43%), P = .821). Significantly less incidence of AKI in patients receiving early diuretics when compared to those in late diuretic group was observed (29% vs 50%, P = .045). Further, those with AKI had a significantly higher fluid balance early in their admission (7.51 mL/kg vs 21.17 mL/kg, P = .027). There was no significant difference in total hospital (13 vs 18 days, P = .233) or ICU length of stay (7 vs 10, P = .095). Table 3 provides a complete summary of patient outcomes.
Table 2.
Patient outcomes.
| ⩽48 h (n = 73) | >48 h (n = 28) | P-value | |
|---|---|---|---|
| Death, n (%) | 28 (38) | 12 (43) | .821 |
| Mechanical ventilation, n (%) | 56 (72) | 21 (75) | .856 |
| Duration of mechanical ventilation, (days) | 4, 5 | 5, 6.5 | .129 |
| Mechanical ventilator free days, (days) | 20 (26) | 16.5 (24) | .460 |
| Negative fluid balance at 24 h | 33 (45.2) | 1 (3.6) | <.001 |
| Negative balance at 24 h at 48 hs | 41 (56.2) | 2 (7.1) | <.001 |
| Negative balance at 24 h at 72 hours | 45 (61.6) | 2 (7.1) | <.001 |
| Fluid balance 24 h (mL) | 398 (2228) | 2460 (2010) | <.001 |
| Fluid balance 48 h (mL) | −173 (3020) | 3612 (3291) | <.001 |
| Fluid balance 72 h (mL) | −139 (4531) | 4370 (4810) | <.001 |
| Fluid balance 24 h (mL/kg) | −1.28, 2.75 | 26.5, 28 | <.001 |
| Fluid balance 48 h (mL/kg) | −1.78, −2.08 | 34.7, 36.5 | <.001 |
| Fluid balance 72 h (mL/kg) | 0.0, −2.39 | 27.7, 70.3 | <.001 |
| Cumulative diuretic dose, (mg) | 320, 593 | 140, 440 | .034 |
| Acute kidney injury, n (%) | 21 (29) | 14 (50) | .045 |
| Hypokalemia, n (%) | 33 (45) | 9 (32) | .233 |
| Hyponatremia | 24 (33) | 7 (25) | .442 |
| Hypocalcemia | 55 (75) | 25 (89) | .122 |
| Vasopressor use, n (%) | 51 (70) | 21 (75) | .609 |
| Total vasopressor requirement, (mg/kg) | 160 | 604 | .280 |
| ICU length of stay, (days) | 7, 9 | 10, 11.75 | .095 |
| Hospital length of stay, (days) | 13, 13.4 | 18, 19 | .233 |
Data are presented as n (%) or mean (standard deviation) unless otherwise noted.
Table 3.
Patient outcomes by LVEF.
| LVEF ⩽40 (n = 54) | LVEF >40 (n = 47) | P-value | |
|---|---|---|---|
| Cumulative diuretic dose (mg) | 597 (882) | 496 (725) | .540 |
| Fluid balance 24 h (mL) | 1035 (2253) | 894 (2480) | .764 |
| Fluid balance 48 h (mL) | 1063 (623) | 623 (3218) | .537 |
| Fluid balance 72 h (mL) | 1060 (3585) | 874 (5100) | .841 |
| Fluid balance 24 h (mL/kg) | 12.6 (29) | 11.7 (30.7) | .881 |
| Fluid balance 48 h (mL/kg) | 15 (51.6) | 9.5 (43.8) | .565 |
| Fluid balance 72 h (mL/kg) | 17.8 (57.7) | 33.1 (173.1) | .540 |
| In-hospital mortality | 24 (44.4) | 16 (34) | .286 |
| Diuretic in 24 h | 34 (63) | 29 (61.7) | .896 |
| Diuretic in 48 h | 41 (75.9) | 32 (68.1) | .380 |
| Acute kidney injury | 18 (33.3) | 17 (36.2) | .765 |
| Vasopressor use | 45 (83.3) | 27 (57.4) | .004* |
| Inotrope use | 12 (22.2) | 9 (19.1) | .531 |
| Mechanical ventilation | 43 (79.6) | 36 (76.6) | .713 |
Abbreviations: LVEF, left-ventricular ejection fraction; SIRS, systemic inflammatory response syndrome; SOFA, sequential organ failure assessment; SD, standard deviation.
Patients stratified by ejection fraction ⩽40% and > 40% showed. To evaluate the role of diuretics alone, an analysis of patients was conducted that excluded any form of RRT. The groups were similar with the exception of a higher total body weight (88.7 vs 74.8 kg, P = .044) and use of outpatient loop diuretics (62.5% vs 35%, P = .017). Early administration of diuretics was associated with a reduced incidence of mechanical ventilation (41 vs 20, P = .01). Reduced duration of mechanical ventilation was also observed in the early diuretic group (4 vs 8 days, P = .018), while mechanical ventilation free days remained the same (20 vs 18, P = .454). Incidence of AKI remained lower in the early diuresis group (26.7 vs 60%, P = .008). These results are summarized in Table 4.
Table 4.
Patient outcomes, no renal replacement therapy subgroup.
| ⩽48 h (n = 56) | > 48 h (n = 20) | P-value | |
|---|---|---|---|
| Age (years) | 65.9 (14.13) | 60.7 (11.8) | .319 |
| Male, n (%) | 30 (53.5) | 12 (60) | .620 |
| Weight (kg) | 88.7 (28.15) | 74.8 (19.7) | .044 |
| LVEF < 40%, n (%) | 32 (57.1) | 9 (45) | .350 |
| SIRS criteria met on admission, n (%) | 45 (80.3) | 17 (85) | .646 |
| SOFA | 8.15 (4.2) | 10.2 (4.45) | .081 |
| Culture positive, n (%) | 30 (53.5) | 11 (55) | .912 |
| Loop diuretic use at home, n (%) | 35 (62.5) | 7 (35) | .017 |
| Death, n (%) | 23 (41.1) | 7 (35) | .633 |
| Mechanical ventilation, n (%) | 41 (73.2) | 20 (100) | .010 |
| Duration of mechanical ventilation, (days) | 4 (8) | 8 (8) | .018 |
| Mechanical ventilator free days, (days) | 20 (27) | 18 (25) | .454 |
| Fluid balance ml/kg/24 h | 5.77 | 34.1 | <.001 |
| Fluid balance ml/kg/48 h | −0.80 | 53.8 | <.001 |
| Fluid balance ml/kg/72 h | −1.31 | 109.1 | .002 |
| Cumulative diuretic dose, (mg) | 310 (550) | 140 (240) | .045 |
| AKI, n (%) | 15 (26.7) | 12 (60) | .008 |
| Hypokalemia, n (%) | 27 (48.2) | 7 (35) | .308 |
| Hyponatremia | 18 (32.1) | 6 (30) | .860 |
| Hypocalcemia | 42 (75) | 18 (90) | .158 |
| ICU length of stay, (days) | 6 (9) | 8 (5) | .315 |
| Hospital length of stay, (days) | 13 (13) | 14 (5) | .471 |
Data are presented as n (%) or mean (SD) unless otherwise noted.
Discussion
In the first study to evaluate the effect of early diuretic therapy in septic patients with HF, early diuretics were associated with reduced fluid balance but not clinical outcomes. However, in a subgroup analysis of patients not requiring renal replacement therapy, diuretic therapy was associated with a reduced need for mechanical ventilation and lower occurrence of AKI.
Positive fluid balance and fluid overload have been repeatedly associated with poor outcomes including increased incidence of AKI, longer hospital length of stay, more days on mechanical ventilation, electrolyte abnormalities, and mortality.14-18 Administration of resuscitation fluids, maintenance IV fluids, and “hidden fluids,” or fluids contained in flushes and diluents for IV drugs all contribute to fluid overload.29-31 In patients with HF, excess fluid balance combined with cardiac dysfunction may have detrimental interrelated consequences. 32 Notably, positive fluid balance may serve as an “intervenable” patient event during an ICU stay that pharmacists can target and prevent, as diuretics represent a readily available strategy. 33
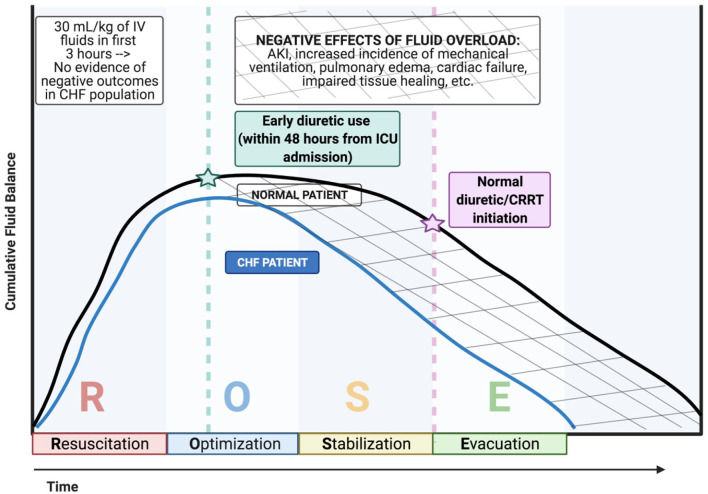
There are no studies about early diuresis in a septic HF population, and the timing of de-resuscitation remains debated. Though the 2021 Surviving Sepsis Guidelines emphasize 30 mL/kg given within the first 3 hours, much less is known regarding when to initiate diuresis. 3 Diuretic protocols have improved outcomes in multiple disease states, including both sepsis and heart failure.22,34,35 Bissell et al. prospectively assessed the implementation of a diuresis protocol in mechanically ventilated patients admitted to the ICU. De-resuscitation with the diuresis protocol was associated with more ventilator and ICU free days and lower mortality. 21 Figure 1 depicts the potential timeline for initiation of resuscitation in practice. Diurese to improve pulmonary dysfunction secondary to volume overload is a common reason for diuretics; however, the underlying septic process that causes mortality is often unrelated to declining respiratory status and occurs primarily from septic processes related to multiorgan failure with failure to maintain adequate blood pressure. 36,37 These disease factors may indicate that only certain patients stand to benefit from this intervention in pathophysiologic grounds.
Figure 1.
ROSE construct including congestive heart failure.
Per the Surviving Sepsis Campaign, 30 mL/kg of IV fluids should be started within 3 h of presentation for all septic patients. There is no evidence that initial resuscitation in CHF patients creates negative outcomes and therefore similar resuscitation inclines are presented for normal and CHF patients. However, normal patients (black line) maintain better cumulative fluid balance through the optimization, stabilization, and evacuation stages compared to CHF patients (blue line). Administration of diuretics within 48 h from ICU admission to septic CHF patients has the potential to reduce negative effects of fluid overload. Illustration created in Biorender.com.
This study has several limitations including its retrospective design, which precludes causal associations between early diuretic therapy and outcomes. Further, as EF measurement was not standardized, the ability to differentia between septic myocardial depression and pre-existing HF was not possible. Additionally, the influence of diuretics on AKI may be obscured due to the high prevalence of acute kidney injury in ICU admission for sepsis and septic shock. Finally, the granular details regarding vasoactive agent requirements at the time of de-resucitation with diuretics were not evaluated which may preclude conclusions about in what timeline of shock de-resucitation should be started.
Conclusion
In conclusion, use of early diuretic therapy appears safe and was associated with lower fluid balance and among patietns not requiring RRT, early diuresis was associated with shorter duration of mechanical ventilation. Further prospective investigation of the interplay of cardiac dysfunction in hemodynamic management of sepsis is warranted.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Conflicts of Interest: Dr. Newsome has received research funding through the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) under Award Numbers UL1TR002378 and KL2TR002381. The remaining authors have disclosed that they do not have any potential conflicts of interest.
ORCID iDs: Aaron M Chase  https://orcid.org/0000-0002-5891-4492
https://orcid.org/0000-0002-5891-4492
Susan E Smith  https://orcid.org/0000-0002-5171-8405
https://orcid.org/0000-0002-5171-8405
Andrea Sikora  https://orcid.org/0000-0003-2020-0571
https://orcid.org/0000-0003-2020-0571
References
- 1. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304-377. [DOI] [PubMed] [Google Scholar]
- 2. Jones TW, Smith SE, Van Tuyl JS, Newsome AS. Sepsis with preexisting heart failure: management of confounding clinical features. J Intensive Care Med. 2021;36:989-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021;47:1181-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miller WL. Fluid volume overload and congestion in heart failure: time to reconsider pathophysiology and how volume is assessed. Circ Heart Fail. 2016;9:e002922. [DOI] [PubMed] [Google Scholar]
- 5. Murphy CV, Schramm GE, Doherty JA, et al. The importance of fluid management in acute lung injury secondary to septic shock. Chest. 2009;136:102-109. [DOI] [PubMed] [Google Scholar]
- 6. Khan RA, Khan NA, Bauer SR, et al. Association between volume of fluid resuscitation and intubation in high-risk patients with sepsis, heart failure, end-stage renal disease, and cirrhosis. Chest. 2020;157:286-292. [DOI] [PubMed] [Google Scholar]
- 7. Leisman DE, Doerfler ME, Ward MF, et al. Survival benefit and cost savings from compliance with a simplified 3-hour sepsis bundle in a series of prospective, multisite, observational cohorts. Crit Care Med. 2017;45:395-406. [DOI] [PubMed] [Google Scholar]
- 8. Duttuluri M, Rose K, Shapiro J, et al. Fluid resuscitation dilemma in patients with congestive heart failure presenting with severe sepsis/septic shock. Am J Respir Crit Care Med. 2016;A7048-A7048. [Google Scholar]
- 9. Liu VX, Morehouse JW, Marelich GP, et al. Multicenter implementation of a treatment bundle for patients with sepsis and intermediate lactate values. Am J Respir Crit Care Med. 2016;193:1264-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seymour CW, Gesten F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376:2235-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kuttab HI, Lykins JD, Hughes MD, et al. Evaluation and predictors of fluid resuscitation in patients with severe sepsis and septic shock. Crit Care Med. 2019;47:1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taenzer AH, Patel SJ, Allen TL, et al. Improvement in mortality with early fluid bolus in sepsis patients with a history of congestive heart failure. Mayo Clin Proc Innov Qual Outcomes. 2020;4:537-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Acharya R, Patel A, Schultz E, et al. Fluid resuscitation and outcomes in heart failure patients with severe sepsis or septic shock: a retrospective case-control study. Plos One. 2021;16:e0256368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koonrangsesomboon W, Khwannimit B. Impact of positive fluid balance on mortality and length of stay in septic shock patients. Indian J Crit Care Med. 2015;19:708-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shen Y, Huang X, Zhang W. Association between fluid intake and mortality in critically ill patients with negative fluid balance: a retrospective cohort study. Crit Care. 2017;21:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ouchi A, Sakuramoto H, Hoshino H, et al. Association between fluid overload and delirium/coma in mechanically ventilated patients. Acute Med Surg. 2020;7:e508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang MP, Jiang L, Zhu B, et al. Association of fluid balance trajectories with clinical outcomes in patients with septic shock: a prospective multicenter cohort study. Mil Med Res. 2021;8:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hawkins WA, Smith SE, Newsome AS, Carr JR, Bland CM, Branan TN. Fluid stewardship during critical illness: a call to action. J Pharm Pract. 2020;33:863-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Malbrain M, Langer T, Annane D, et al. Intravenous fluid therapy in the perioperative and critical care setting: executive summary of the international fluid academy (IFA). Ann Intensive Care. 2020;10:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Malbrain M, Van Regenmortel N, Saugel B, et al. Principles of fluid management and stewardship in septic shock: it is time to consider the four D’s and the four phases of fluid therapy. Ann Intensive Care. 2018;8:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lal A, Garces JPD. Physiologic approach to diuresis in de-resuscitation phase in intensive care. Crit Care. 2020;24:1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bissell BD, Laine ME, Thompson Bastin ML, et al. Impact of protocolized diuresis for de-resuscitation in the intensive care unit. Crit Care. 2020;24:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dhondup T, Tien JC, Marquez A, Kennedy CC, Gajic O, Kashani KB. Association of negative fluid balance during the de-escalation phase of sepsis management with mortality: a cohort study. J Crit Care. 2020;55:16-21. [DOI] [PubMed] [Google Scholar]
- 24. Ambrosy AP, Cerbin LP, Armstrong PW, et al. Body weight change during and after hospitalization for acute heart failure: patient characteristics, markers of congestion, and outcomes: findings from the ASCEND-HF trial. JACC Heart Fail. 2017;5:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. J Am Coll Cardiol. 2013;62:e147-239. [DOI] [PubMed] [Google Scholar]
- 26. Shen Y, Zhang W, Shen Y. Early diuretic use and mortality in critically ill patients with vasopressor support: a propensity score-matching analysis. Critical Care. 2019;23:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McMurray JJ. Clinical practice. Systolic heart failure. N Engl J Med. 2010;362:228-238. [DOI] [PubMed] [Google Scholar]
- 28. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179-184. [DOI] [PubMed] [Google Scholar]
- 29. Carr JR, Hawkins WA, Newsome AS, et al. Fluid Stewardship of Maintenance Intravenous Fluids. J Pharm Pract. Published online April 8, 2016. doi: 10.1177/0897190021100826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Branan T, Smith SE, Newsome AS, Phan R, Hawkins WA. Association of hidden fluid administration with development of fluid overload reveals opportunities for targeted fluid minimization. SAGE Open Med. 2020;8:2050312120979464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gamble KC, Sikora SS, Newsome A, Bland CM, Branan T, Hawkins WA. Hidden fluids in plain sight: identifying intravenous medication classes as contributors to intensive care unit fluid intake. Hosp Pharm. 2021;00185787211016339. doi: 10.1177/00185787211016339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pellicori P, Kaur K, Clark AL. Fluid management in patients with chronic heart failure. Card Fail Rev. 2015;1:90-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Olney WJ, Chase AM, Hannah SA, Smith SE, Newsome AS. Medication regimen complexity score as an indicator of fluid balance in critically Ill patients. J Pharm Pract. 2021;897190021999792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Wiedemann HP, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564-2575. [DOI] [PubMed] [Google Scholar]
- 35. Barsuk JH, Gordon RA, Cohen ER, et al. A diuretic protocol increases volume removal and reduces readmissions among hospitalized patients with acute decompensated heart failure. Congest Heart Fail. 2013;19:53-60. [DOI] [PubMed] [Google Scholar]
- 36. Ketcham SW, Sedhai YR, Miller HC, et al. Causes and characteristics of death in patients with acute hypoxemic respiratory failure and acute respiratory distress syndrome: a retrospective cohort study. Crit Care. 2020;24:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stapleton RD, Wang BM, Hudson LD, Rubenfeld GD, Caldwell ES, Steinberg KP. Causes and timing of death in patients with ARDS. Chest. 2005;128(2):525-532. [DOI] [PubMed] [Google Scholar]