Abstract
Objective
Insulin dysregulation is a hallmark of equine metabolic syndrome (EMS) and increases the risk for development of laminitis. Accurate diagnosis of insulin dysregulation is crucial for implementation of preventative strategies in this population. The objective was to assess the effects of dexamethasone administration on insulin and glucose dynamics in light-breed horses and assess the agreement of various diagnostic tests for insulin dysregulation [basal [insulin] (BI), oral sugar test (OST), and combined glucose-insulin test (CGIT)].
Animal
Fourteen adult light-breed horses.
Procedure
Prospective, experimental study to assess insulin and glucose dynamics by performing basal insulin, OST, and CGIT before (baseline) and post-dexamethasone administration (0.08 mg/kg, PO, q24h) for 7 d. Insulin and glucose dynamics were assessed by the BI, OST, CGIT, and insulin sensitivity proxy measurements (RISQI, QUICKI, FGIR, HOMA-IR, IG) at the baseline and post-dexamethasone time points.
Results
The OST area under the insulin and glucose curves were increased following dexamethasone treatment (P < 0.001 and P < 0.01, respectively). Basal insulin, OST [insulin] at 60 min and CGIT [insulin] at 45 min were increased at the post-dexamethasone time point (P < 0.001, < 0.001, and < 0.01). Similarly, time spent in the positive glucose phase during the CGIT was longer at the post-dexamethasone time point (P < 0.001). The proxy measurements for insulin sensitivity (RISQI, QUICKI, FGIR) were decreased (P < 0.01) and the proxy measurements for insulin resistance (HOMA-IR) and β-cell function (IG) were increased after dexamethasone administration (P < 0.01). More horses were classified with following dexamethasone administration, based on the diagnostic criteria for basal insulin (P = 0.03), OST (P = 0.01), and CGIT (P < 0.01). Kappa coefficients, measuring agreement between basal insulin, OST, and CGIT, showed none to moderate agreement at the baseline time point.
Conclusion
Dexamethasone administration at 0.08 mg/kg, PO, q24h for 7 d worsened insulin dysregulation in adult light-breed horses based on findings of a basal insulin, OST, CGIT, and insulin sensitivity proxy measurements. There was none to moderate agreement between the basal insulin, OST, CGIT for the diagnosis of insulin dysregulation.
Clinical relevance
Horses administered dexamethasone at a dose of 0.08 mg/kg, PO, q24h for 7 d should be considered insulin dysregulation and appropriate preventative strategies should be implemented. The variability of diagnostic performance of common tests for insulin dysregulation (basal insulin, OST, CGIT) may affect clinical decisions; therefore, performing multiple tests, including proxy measurements, may improve diagnostic accuracy of insulin dysregulation.
Résumé
Objectif
La dysrégulation de l’insuline est une caractéristique du syndrome métabolique équin (EMS) et augmente le risque de développement de la fourbure. Un diagnostic précis de la dysrégulation de l’insuline est crucial pour la mise en oeuvre de stratégies préventives dans cette population. L’objectif était d’évaluer les effets de l’administration de dexaméthasone sur la dynamique de l’insuline et du glucose chez les chevaux de race légère et d’évaluer la concordance de divers tests de diagnostic pour le dérèglement de l’insuline [insuline basale] (BI), test de sucre oral (OST) et un test glucose-insuline combiné (CGIT).
Animal
Quatorze chevaux adultes de race légère.
Procédure
Étude prospective et expérimentale pour évaluer la dynamique de l’insuline et du glucose en effectuant l’insuline basale, l’OST et le CGIT avant (valeur de base) et après l’administration de dexaméthasone (0,08 mg/kg, PO, q24h) pendant 7 jours. La dynamique de l’insuline et du glucose a été évaluée par les mesures indirectes de BI, de l’OST, du CGIT et de la sensibilité à l’insuline (RISQI, QUICKI, FGIR, HOMA-IR, IG) aux points temporels de base et post-dexaméthasone.
Résultats
La zone OST sous les courbes d’insuline et de glucose a augmenté après le traitement à la dexaméthasone (P < 0,001 et P < 0,01, respectivement). L’insuline basale, l’OST [insuline] à 60 minutes et le CGIT [insuline] à 45 minutes ont augmenté au point temporel post-dexaméthasone (P < 0,001, < 0,001 et < 0,01). De même, le temps passé dans la phase de glucose positif pendant le CGIT était plus long au moment post-dexaméthasone (P < 0,001). Les mesures indirectes de la sensibilité à l’insuline (RISQI, QUICKI, FGIR) ont diminué (P < 0,01) et les mesures indirectes de la résistance à l’insuline (HOMA-IR) et de la fonction des cellules β (IG) ont augmenté après l’administration de dexaméthasone (P < 0,01). Plus de chevaux ont été classés avec l’administration suivante de dexaméthasone, sur la base des critères de diagnostic de l’insuline basale (P = 0,03), OST (P = 0,01) et CGIT (P < 0,01). Les coefficients Kappa, mesurant la concordance entre l’insuline basale, l’OST et le CGIT, ont montré une concordance nulle à modérée au point de référence.
Conclusion
L’administration de dexaméthasone à 0,08 mg/kg, PO, toutes les 24 h pendant 7 jours a aggravé la dysrégulation de l’insuline chez les chevaux adultes de race légère d’après les résultats d’une insuline basale, d’OST, de CGIT et de mesures indirectes de la sensibilité à l’insuline. Il n’y avait aucun accord à modéré entre l’insuline basale, l’OST, le CGIT pour le diagnostic de dysrégulation de l’insuline.
Pertinence clinique
Les chevaux ayant reçu de la dexaméthasone à une dose de 0,08 mg/kg, PO, q24h pendant 7 jours doivent être considérés comme ayant un dérèglement de l’insuline et des stratégies préventives appropriées doivent être mises en oeuvre. La variabilité des performances diagnostiques des tests courants de dysrégulation de l’insuline (insuline basale, OST, CGIT) peut affecter les décisions cliniques; par conséquent, la réalisation de plusieurs tests, y compris des mesures indirectes, peut améliorer la précision du diagnostic du dérèglement de l’insuline.
(Traduit par Dr Serge Messier)
Introduction
Insulin dysregulation, a hallmark feature of equine metabolic syndrome (EMS), is associated with an increased risk for development of laminitis (1). The underlying pathophysiology is not completely understood, but induction of prolonged hyperinsulinemia using the euglycemic-hyperinsulinemic clamp (EHC) has induced laminitis in previously healthy equids (2,3). Glucocorticoids are commonly used in equine practice for treatment of inflammatory and immune-mediated disorders; however, corticosteroids can influence insulin and glucose dynamics, leading to development of insulin dysregulation in otherwise healthy horses (4,5). The administration of glucocorticoids has also been associated with development of laminitis (6), and may be more likely to occur in horses that are systemically ill while concurrently receiving glucocorticoid therapy (7–9). Laminitis induced through the EHC and glucocorticoid-induced laminitis results in similar histopathologic findings, characterized by separation of the dermo-epidermal junction as well as lengthening and attenuation of the primary and secondary lamellae (3,6), thus supporting a possible shared mechanism of laminitis induction.
The diagnostic accuracy of insulin dysregulation, regardless of the cause, is crucial in identifying horses at-risk for development of laminitis and other metabolic derangements associated with insulin dysregulation, to implement preventative treatment strategies in at-risk equids. There are several diagnostic tests used to diagnose insulin dysregulation in horses, but the sensitivity, repeatability, and the agreement among these tests is highly variable (10–12). Gold standard tests for the diagnosis of insulin dysregulation, such as the EHC and frequently sampled insulin-modified intravenous glucose tolerance test (FSIGTT), are technical and cumbersome to perform in traditional field settings. However, fasting basal insulin and glucose concentrations are easy to perform and enable calculation of insulin sensitivity proxy measurements to further evaluate insulin and glucose dynamics. Proxy measurements are commonly used in human medicine to assess insulin sensitivity, insulin resistance, and pancreatic β-cell function and have been well-validated when compared to gold standard testing in humans (13); however, specific diagnostic cut-off values have not been validated in horses. Furthermore, values obtained from a single baseline blood glucose and insulin concentration measurement may not consistently identify horses with insulin dysregulation, as external factors such as length of fasting (14), sampling time (15), stress-induced cortisol release (16), and concurrent medications (17) can affect insulin and glucose concentrations. The oral sugar test (OST) was designed to evaluate a horse’s glucose-induced insulin response following oral administration of non-structural carbohydrates, thereby also assessing the enteroinsular axis or incretin response (18,19). The combined glucose and insulin test (CGIT), another dynamic test, was designed to assess peripheral insulin sensitivity through intravenous administration of glucose and insulin (12). Performance of these dynamic tests is extremely variable among cohorts (10,20,21), making continued evaluation of these testing strategies warranted.
The objective of this study was to evaluate insulin and glucose responses in adult light-breed horses before and after dexamethasone administration (0.08 mg/kg, PO, q24h for 7 d) through assessment of baseline blood [insulin] and [glucose], calculation of insulin sensitivity proxy measurements, and evaluation of the OST and CGIT at 2 time points, baseline and post-dexamethasone. A second objective was to compare the degree of agreement between the basal insulin and dynamic tests (OST, CGIT) in the same cohort of horses prior to administration of dexamethasone. We hypothesized that administration of dexamethasone for 1 wk would induce or exacerbate insulin dysregulation as assessed by baseline blood [insulin] and [glucose], insulin sensitivity proxy measurements, and results of dynamic testing for insulin dysregulation (OST, CGIT). In addition, we hypothesized that for the diagnosis of insulin dysregulation, there would be low agreement between the basal insulin and dynamic testing (OST, CGIT) based on commonly used diagnostic criteria.
Materials and methods
Experimental design
The experimental procedures in this study were approved by the OSU Institutional Animal Care and Use Committee (Protocol 2014A00000029) in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Fourteen adult light-breed horses owned by the Ohio State University College of Veterinary Medicine and housed at the OSU Veterinary Medical Center (VMC) were included in this prospective experimental study. Inclusion criteria for the study included age > 4 and ≤ 15 y and an endogenous [adrenocorticotrophic hormone (ACTH)] (Animal Health Diagnostic Center, Cornell University, Ithaca, New York, USA) < 35 pg/mL tested in May. The horses were housed in individual stalls at the OSU VMC and fed grass hay and given ad libitum access to water for a 5-day acclimation period prior to the start of the experimental protocols. The grass hay fed was submitted for nutritional analysis (Equi Analytical, Ithaca, New York, USA) (Table 1).
Table 1.
Proximate analysis of forage consumed by study subjects.
| As sampled | Dry matter | |
|---|---|---|
| Digestible energy (Mcal/kg) | 1.92 | 2.05 |
| Crude protein (%) | 8.7 | 9.3 |
| Water-soluble carbohydrate (%) | 8.5 | 9.1 |
| Non-fiber carbohydrate (%) | 16.9 | 18.1 |
Equi-Analytical Laboratory Services, Ithaca, New York, USA.
Insulin and glucose dynamics were assessed at 2 time points, baseline and post-dexamethasone, and testing occurred over 2 d. Baseline sampling was performed following the acclimation period; a basal insulin and OST were performed on Day 5, and a CGIT was performed on Day 6 of the study. Then, all horses were treated with dexamethasone (0.08 mg/kg, PO, q24h), beginning on Day 7, for 7 d. Testing for the post-dexamethasone timepoint occurred on Days 14 (basal insulin, OST) and 15 (CGIT). All testing took place in July to August 2019.
Oral sugar test (OST)
The OST was performed as described (22). The horses were fasted for approximately 12 h before the start of the test. Briefly, approximately 1 h prior to the start of the study, an IV catheter (Abbocath-T Catheter 14 G × 5.5″; Abbott Animal Health, Chicago, Illinois, USA) was placed in a jugular vein for blood collection. Catheter patency was maintained with heparinized saline irrigation after blood collection and every 6 h thereafter for 36 h. Prior to sample collection, a minimum of 10 mL of blood was collected from the IV catheter and discarded. A baseline blood sample (T0) was collected for blood [glucose] and [insulin] measurements. Then, light corn syrup (Karo Light Corn Syrup; ACH Food Companies, Inc., Oakbrook, Illinois, USA), 0.15 mL/kg, PO to deliver about 150 mg/kg of digestible carbohydrates. Subsequent blood samples were collected at 30, 60, 90, 120, 150, 180, and 240 min post-corn syrup administration to determine blood [glucose], with samples at 60, 120, and 150 min also used to determine plasma [insulin].
Combined glucose and insulin test (CGIT)
The horses were fasted as for the OST. The CGIT was performed as described (12). Briefly, the IV catheter placed the previous day for the OST was used for blood sample collection, and patency was maintained with heparinized saline irrigation. Prior to sample collection, a minimum of 10 mL of blood was collected from the IV catheter and discarded before each sample collection. Baseline (T0) blood samples were collected to determine blood [glucose] and [insulin]. Then, a 50% dextrose solution (VetOne, MWI Animal Health, Boise, Idaho, USA), 150 mg/kg, IV immediately followed by regular insulin (Humulin R; Eli Lilly and Company, Indianapolis, Indiana, USA), 0.1 U/kg in the opposite jugular vein via a 19-gauge winged infusion (SURFLO Winged infusion set; Terumo Medical, Somerset, New Jersey, USA) set, over 1 to 2 min. The infusion set was flushed with 10 mL of heparinized saline and removed. Additional blood samples were collected at 1, 5, 15, 25, 35, 45, 60, 75, 90, 105, 120, 135, and 150 min after dextrose and insulin administration to determine blood [glucose], with samples at 45, 90, and 120 min also used to determine plasma [insulin].
Sample analyses
Blood samples taken during the OST and CGIT were collected in ethylenediaminetetraacetic acid (EDTA) (K2 EDTA Vacutainer tubes; Becton Dickinson, Franklin Lakes, New Jersey, USA) and silicone-coated tubes (Silicone-coated Vacutainer tubes; Becton Dickinson) and immediately placed on ice. A hand-held glucometer (AlphaTRAK blood glucose monitoring system meter; Zoetis, Kalamazoo, Michigan, USA), previously validated for equine whole blood (23), was used to measure blood [glucose] at the time of collection. All blood samples were centrifuged within 6 h after collection; serum and plasma were aliquoted and stored at −80°C until analysis. Plasma [insulin] was measured using a commercial enzyme-linked immunosorbent assay (ELISA) (Insulin ELISA 07M-60102; MP Biomedicals, Solon, Ohio, USA) validated for use in horses (24).
Data analysis
A power calculation was performed using statistical software (G*Power 3.1; Heinrich Heine Universitat, Düsseldorf, Germany). To determine differences between insulin and glucose parameters and proxy measurements using a paired Student’s t-test or Wilcoxon signed-rank test, a minimum sample size of 6 horses was required based on a power calculation of 0.95 and α = 0.05. To assess changes in categorical classification of insulin dysregulation using a Fisher’s Exact test, a minimum sample size of 14 horses, with 7 horses in each group, was required based on a power calculation of 0.8 and a pre-test probability of 0.15 and post-test probability of 0.85.
The basal [insulin] and [glucose] measurements were obtained from samples collected at Time 0 of the OST at both time points: baseline and post-dexamethasone. The area under the curve for glucose (AUCgluc0–240), area under the curve for insulin (AUCinsulin0–150), and the insulin concentration at 60 min ([Insulin]60) were calculated for the OST at both time points. The CGIT parameters calculated included insulin concentration at 45 min ([Insulin]45), glucose concentration at 45 min ([Glucose]45), and the positive phase duration of the glucose curve (PP-Dglu) at both time points. Outliers were identified using the ROUT method with a Q of 1% and removed prior to statistical analysis (25). Quantitative variables were assessed for normality using the D’Agostino & Pearson omnibus normality test. The basal insulin, OST(AUCgluc0–240), OST(AUCinsulin0–150), OST[Insulin]60, and CGIT[Insulin]45 were normally distributed and are displayed as mean ± standard deviation (SD) (Table 2). The basal [glucose] and the (PP-Dgluc) were not normally distributed and are displayed as median [interquartile range (IQR)]. A paired Student’s t-test was used to compare time points of normally distributed data (BI, OST(AUCgluc0–240), OST(AUCinsulin0–150), OST[Insulin]60, and CGIT[Insulin]45) and a Wilcoxon matched-pairs signed rank test was used to compare time points of non-normally distributed data (basal [glucose], PP-Dgluc). Statistical significance was set at P < 0.05.
Table 2.
Effects of dexamethasone administration on fasting basal insulin and glucose concentrations, parameters of the CGIT, OST, and insulin sensitivity proxy measurements.
| Effect measured | Measurement | Baseline | Post-dexamethasone |
|---|---|---|---|
| Basal insulin | Insulin (μIU/mL) | 13.10 ± 6.90a | 50.9 ± 27.2*** |
| Basal glucose | Glucose (mg/dL) | 94 (85 to 123)b | 128 (109 to 184)*** |
| OST(AUCInsulin0–150) | Insulin (μIU/mL × min) | 3770 ± 3119a | 14 666 ± 6510*** |
| OST(AUCgluc0–240) | Glucose (mg/dL × min) | 4037 ± 3321a | 8851 ± 5326** |
| OST[Insulin]60 | Insulin (μIU/mL) | 44.87 ± 25.68a | 151.20 ± 73.34*** |
| CGIT[Insulin]45 | Insulin (μIU/mL) | 28.23 ± 11.46a | 101.3 ± 72.80** |
| CGIT(PP-Dgluc) | Time (min) | 46 (15 to 150)b | 160 (75 to 180)*** |
| Insulin sensitivity | QUICKI | 0.32 ± 0.03a | 0.26 ± 0.03*** |
| RISQI | 0.29 ± 0.08a | 0.15 ± 0.06*** | |
| FGIR | 8.90 ± 4.38a | 3.26 ± 2.13*** | |
| Insulin resistance | HOMA-IR | 3.24 ± 1.81a | 17.31 ± 10.93*** |
| β-cell function | MIRG | 2.86 ± 2.32a | 6.09 ± 1.92 |
| IG | 0.13 ± 0.07a | 0.38 ± 0.18*** |
Indicates mean ± SD.
Indicates median and range.
Different from baseline time point;
P < 0.01;
P < 0.001.
SD — Standard deviation; AUCInsulin0–150 — Area under the curve insulin 0 to 150 min; AUCgluc0–240 — Area under the curve glucose 0 to 240 min; [Insulin]60 — Insulin concentration at 60 min; [Insulin]45 — Insulin concentration at 45 min; PP-Dgluc — Positive phase duration glucose; QUICKI — Quantitative insulin sensitivity check index; RISQI — Reciprocal of the square root of insulin; FGIR — Fasting glucose-to-insulin ratio; HOMA-IR — Homeostasis model of assessment for insulin resistance; MIRG — Modified insulin-to-glucose ratio; IG — Insulin-to-glucose ratio.
To further assess the effects of dexamethasone on insulin and glucose dynamics, proxy measurements of insulin sensitivity were calculated for all horses at the baseline (n = 14) and post-dexamethasone (n = 14) time points, using the following formulae:
The [glucose] and [insulin] measurements obtained from the Time 0 sample of the OST testing days were used for proxy calculations. Outliers were identified using the ROUT method with a Q of 1% and removed prior to statistical analysis (25). As a result, 1 horse was removed from statistical analysis of basal blood [glucose], plasma [insulin], MIRG, and IG. Assessment of normality was performed using the D’Agostino and Pearson omnibus test. The proxy measurement data and basal plasma [insulin] were normally distributed. A Student’s paired t-test was performed to evaluate differences in proxy measurements between the baseline and post-dexamethasone time points. Commercial statistical software (GraphPad Prism 8; GraphPad Software, La Jolla, California, USA) was used for all data analyses.
A positive diagnosis of insulin dysregulation was determined based on a basal insulin > 20 μIU/mL, OST[Insulin]60 > 60 μIU/mL, OST[Insulin]60 > 45 μIU/mL, CGIT[Gluc]45 > baseline [glucose], and CGIT[Insulin]45 > 100 μIU/mL at each time point. A Fisher’s Exact test was used to assess the categorical classification of insulin dysregulation between baseline and post-dexamethasone time points according to the previously mentioned diagnostic criteria for basal insulin, OST, and CGIT. In addition, agreement of categorical classification of insulin dysregulation at the baseline time point between BI > 20 μIU/mL and OST[Ins]60 > 60 μIU/mL, OST[Ins]60 > 45 μIU/mL, CGIT[Gluc]45 > baseline[glucose], and CGIT[Ins]45 > 100 μIU/mL, and BI > 20 μIU/mL performed 24 h apart were assessed using Cohen’s Kappa (0.8 to 1.0 indicates almost perfect agreement, 0.6 to 0.8 substantial agreement, 0.2 to 0.4 fair agreement, 0.0 to 0.2 slight agreement, and < 0.0 poor agreement).
Results
Fourteen adult, light-breed horses were used in the study. The study population consisted of 9 mares and 5 geldings of various breeds (3 Warmbloods, 3 American Quarter Horses, 3 Thoroughbreds, 2 Trakehners, 1 American Paint Horse, 1 Standardbred, and 1 grade horse). The endogenous [ACTH] was within a normal seasonally adjusted reference range for all horses, with a mean of 19.0 ± 5.4 pg/mL. The mean age and weight of the horses were 12 ± 3 y and 527 ± 67.8 kg.
The baseline plasma [insulin] and the blood [glucose] concentrations were significantly increased at the post-dexamethasone time point compared to baseline (P < 0.00 and < 0.001, respectively). The OST parameters measured of AUCinsulin0–150, AUCgluc0–240, and [insulin]60 were increased at the post-dexamethasone time point compared to baseline (P < 0.001, < 0.01, and < 0.001, respectively; Table 2). Similarly, the CGIT parameters measured of [insulin]45 and PP-Dgluc were increased at the post-dexamethasone time point compared to baseline (P < 0.01 and < 0.001, respectively; Table 2). In addition, the proxy measurements of insulin sensitivity (QUICKI, RISQI, FGIR) were all decreased at the post-dexamethasone time point compared to the baseline timepoint (P < 0.01, < 0.01, and < 0.01, respectively; Table 2). The proxy measurement for insulin resistance (HOMA-IR) was increased at the post-dexamethasone time point compared to the baseline time point (P < 0.01). The IG, a proxy measurement for β-cell function, was elevated at the post-dexamethasone time point compared to the baseline time point (P < 0.1). However, the MIRG, another proxy measurement for β-cell function, was not different between time points (P = 0.30).
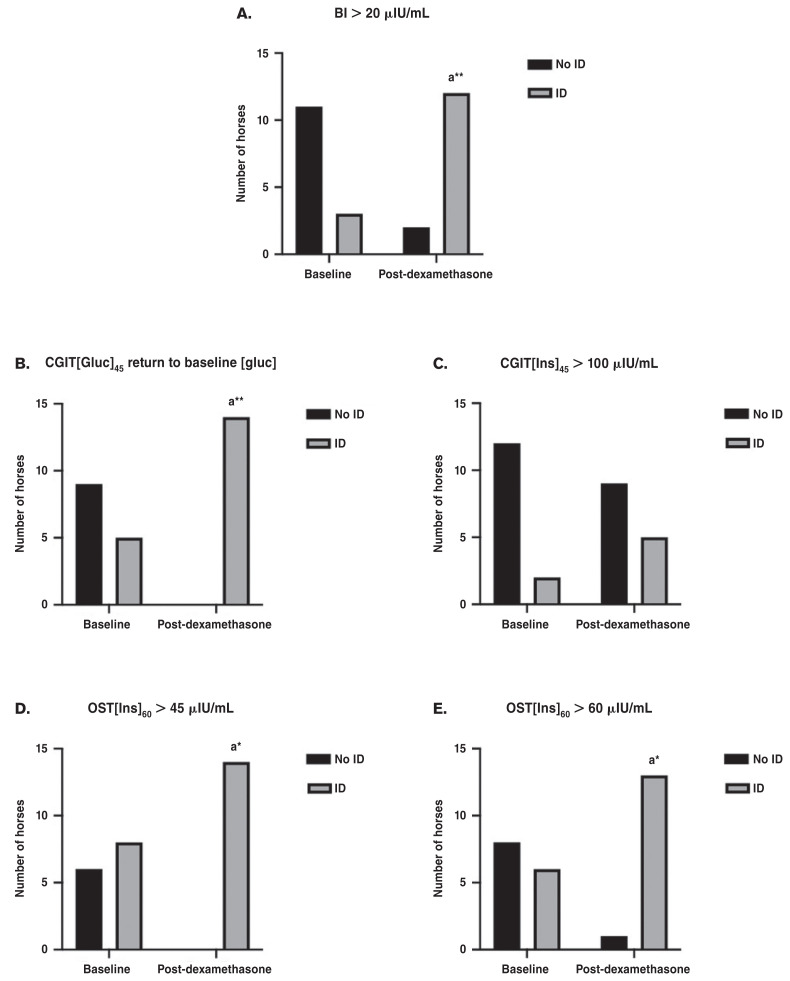
The diagnosis of insulin dysregulation was made at each time point according to the diagnostic criteria for basal insulin, OST, and CGIT. At the baseline time point, a basal insulin > 20 μIU/mL diagnosed 4 horses as insulin dysregulation, but at the post-dexamethasone time point, 13 horses were classified as insulin dysregulation using this test and cutoff. Similarly, at the baseline time point, an OST[Ins]60 > 60 μIU/mL diagnosed 6 horses as insulin dysregulation, whereas at the post-dexamethasone time point, 13 horses met the criteria for insulin dysregulation diagnosis. At the baseline time point, an OST[Ins]60 > 45 μIU/mL identified 8 horses as insulin dysregulation, which increased to 14 horses at the post-dexamethasone basal insulin time point. According to a CGIT[Gluc]45 return to baseline [glucose], 5 horses were diagnosed with insulin dysregulation at the baseline time point, and all 14 horses were classified as insulin dysregulation at the post-dexamethasone time point. However, based on CGIT[Ins]45 > 100 μIU/mL, only 2 horses were classified with insulin dysregulation at the baseline time point, which only increased to 5 horses classified with insulin dysregulation at the post-dexamethasone time point. When comparing changes in the categorical classifications of insulin dysregulation, dexamethasone administration for 7 d at 0.08 mg/kg, PO, q24h resulted in significantly more horses being classified as insulin dysregulation based on basal insulin > 20 μIU/mL (P = 0.03), OST[Ins]60 > 60 μIU/mL (P = 0.01), OST[Ins]60 > 45 μIU/mL (P = 0.01), and CGIT[Gluc]45 return to baseline [glucose] (P < 0.01), but not for CGIT[Ins]45 > 100 μIU/mL (P = 0.38) (Figure 1).
Figure 1.
Changes in categorical classification of insulin dysregulation following treatment with dexamethasone. Graph A demonstrates that significantly more horses were classified as insulin dysregulation using the basal insulin following dexamethasone treatment. Graph B demonstrates that significantly more horses were classified as insulin dysregulation using the criteria of CGIT[Gluc]45 returning to baseline blood [glucose]. Graph C demonstrates no statistical difference in the number of horses classified as insulin dysregulation based on the CGIT[Ins]45 > 100 μIU/mL. Graph D demonstrates that significantly more horses were diagnosed with insulin dysregulation based on the OST[Ins]60 > 45 μIU/mL. Graph E demonstrates that significantly more horses were diagnosed with insulin dysregulation based on OST[Ins]60 > 60 μIU/mL.
a Indicates significance from baseline time point; *P < 0.05.
AUCgluc0–240 — Area under the glucose curve from 0 to 240 min; AUCins0–150 — Area under the insulin curve from 0 to 150 min; [Ins]60 — Insulin concentration at 60 min; AUCgluc0–150 — Area under the glucose curve from 0 to 150 min; PP-Dgluc — Positive phase duration of the glucose curve; [Ins]45 — Insulin concentration at 45 min.
Cohen’s Kappa coefficients were calculated to compare the degree of agreement between > 20 μIU/mL and the standard diagnostic criteria for the OST and CGIT (Table 3). The agreement between 2 basal insulin assessments performed 24 h apart was moderate (0.444). Similarly, the agreement between basal insulin and the OST analysis (OST[Ins]60 > 60 μIU/mL, OST[Ins]60 > 45 μIU/mL) was also moderate (0.512, 0.444, respectively). However, the agreement between basal insulin and the CGIT analysis was poor (CGIT[Gluc]45 return to baseline [glucose]; −0.026) and slight (CGIT[Ins]45 > 100 μIU/mL; 0.122), depending on the diagnostic criteria.
Table 3.
Cohen’s Kappa degree of agreement between indices used to diagnose insulin dysregulation based on basal insulin, OST, and CGIT.
| Agreement with basal insulin > 20 μIU/mL | Cohen’s Kappa | Level of agreement |
|---|---|---|
| OST[Ins]60 > 60 μIU/mL | 0.512 | Moderate |
| OST[Ins]60 > 45 μIU/mL | 0.444 | Moderate |
| CGIT[Gluc]45 > baseline [glucose] | −0.026 | Poor |
| CGIT[Ins]45 > 100 μIU/mL | 0.122 | Slight |
| Basal insulin > 20 μIU/mL performed 24 h apart | 0.444 | Moderate |
OST — Oral sugar test; CGIT — Combined glucose and insulin test.
Discussion
The current study demonstrated that dexamethasone administered at 0.08 mg/kg, PO, q24h for 7 d induced worsening insulin dysregulation as assessed by the basal insulin, OST, CGIT, and insulin sensitivity proxy measurements. We also performed an evaluation of level of agreement between frequently used tests for insulin dysregulation (basal insulin, OST, CGIT) and detected variable agreement between tests for the diagnosis of insulin dysregulation at the baseline time point.
Treatment with dexamethasone for 7 d increased basal blood [glucose] (P < 0.01) and basal plasma [insulin] (P < 0.01), demonstrating an alteration in insulin and glucose dynamics. This finding differed from a previous study, which reported dexamethasone administration (0.08 mg/kg, IV, q48h) to significantly increase [insulin], but not [glucose] after 7 d of treatment (5). The AUCglucose and AUCinsulin calculated from the OST positively correlate to those achieved by the FSIGTT and therefore can be used as an indirect measurement of insulin sensitivity (22). The OST(AUCInsulin0–150) and OST(AUCglucose0–240) were increased following treatment with dexamethasone (P < 0.001 and < 0.01, respectively), thus indicating a progressive decrease in insulin sensitivity following dexamethasone administration. Similarly, the OST[insulin]60 was increased at the post-dexamethasone time point (P < 0.001), further demonstrating an exacerbation of insulin dysregulation following dexamethasone administration. Furthermore, the CGIT[insulin]45 was increased subsequent to dexamethasone administration (P < 0.01), indicating a decrease in tissue insulin sensitivity. Moreover, the CGIT(PP-Dgluc) remained longer in the positive phase of the glucose curve (P < 0.001), thus corroborating the findings that dexamethasone worsens insulin dysregulation by decreasing peripheral tissue insulin sensitivity. Therefore, administration of dexamethasone at 0.08 mg/kg, PO, q24h for 7 d can worsen insulin dysregulation in adult light-breed horses, based on insulin and glucose dynamics assessed by the basal insulin, OST, and CGIT.
Proxy measurements for insulin sensitivity have been widely used in human clinical practice and epidemiologic studies for assessment of insulin dysregulation (13). Proxy measurements were assessed in this study to evaluate effects of dexamethasone administration on various aspects of insulin dysregulation (insulin sensitivity, insulin resistance, and pancreatic β-cell response). The proxy measurements for insulin sensitivity (QUICKI, RISQI, FGIR) were all decreased following dexamethasone treatment (P < 0.001). Similarly, in a previous study, there was a significantly reduced RISQI in horses treated with dexamethasone for 7, 14, and 21 d compared to control horses (5). The proxy measurement for insulin resistance (HOMA-IR) was increased following dexamethasone administration for 7 d (P < 0.001), indicating an exacerbation of insulin resistance with treatment. The IG, a proxy measurement assessing β-cell responsiveness, was increased following dexamethasone treatment (P < 0.001). However, the MIRG, which is also a proxy measurement assessing β-cell responsiveness, increased following dexamethasone treatment; however, this difference was not significant (P = 0.59). Again, these findings supported a previous report of a significantly increased MIRG value following dexamethasone treatment for 21 d (5). Therefore, we inferred that dexamethasone induced a state of compensated insulin resistance, as markers of insulin sensitivity decreased, whereas β-cell responsiveness increased.
More horses were classified as having insulin dysregulation following dexamethasone treatment based on a BI cut-off value of > 20 μIU/mL (P = 0.03) (1). Similarly, 2 diagnostic criteria were used to make the diagnosis of insulin dysregulation based on the OST: an [Ins]60 > 60 μIU/mL and an [Ins]60 > 45 μIU/mL. Using both the original cut-off value of [insulin] being > 60 μIU/mL (26) and a newly recommended, more sensitive cut-off value of [insulin] > 45 μIU/mL (27) at 60 min, more horses were classified with insulin dysregulation following 7 d of dexamethasone treatment (P = 0.01). These findings were further supported by more horses being classified as insulin dysregulation based on the CGIT criteria of the blood [glucose] returning to baseline by 45 min (P < 0.01) (28). In contrast, when assessing for insulin dysregulation based on the CGIT criteria of an [Ins]45 > 100, there was no significant difference in the number of horses diagnosed with insulin dysregulation following dexamethasone treatment. The discrepancy in the CGIT-based diagnoses could be due to development of uncompensated insulin resistance, in which blood [glucose] remains elevated, accompanied by a lower peak [insulin] (1). We inferred that the degree of exacerbation of insulin dysregulation caused by the administration of dexamethasone for 1 wk can lead to a significant proportion of horses becoming diagnostically insulin dysregulation. Consequently, horses administered dexamethasone should be considered at-risk for development of insulin dysregulation.
Our findings demonstrated that dexamethasone (0.08 mg/kg, PO, q24h) administered for 7 d exacerbated insulin dysregulation, consistent with other studies with similar findings following glucocorticoid administration (various dosages and routes) (4,5). The exact mechanism by which glucocorticoids induce insulin dysregulation is not yet completely understood; however, it is likely multifactorial. Humans treated with long-term glucocorticoids often develop Cushingoid features, such as abdominal obesity, dyslipidemia, cervical fat deposits, insulin resistance, and hyperglycemia (29). Glucocorticoids increase hepatic gluconeogenesis by activating genes regulating carbohydrate metabolism, such as phosphoenolpyruvate carboxykinase (PEPCK), which enhances gluconeogenesis within the liver (30). In addition, glucocorticoids negatively impact secretion, proliferation, and survival of pancreatic β-cells (29). Glucocorticoids induce insulin resistance in adipose tissue through decreased phosphorylation of insulin receptor substrate-1 (IRS-1), which directly impacts insulin signaling following initial activation of the insulin receptor (31). In addition, glucocorticoids decrease insulin-induced glucose uptake within adipocytes due to decreased expression of the GLUT4 transporter and decreased translocation of GLUT4 to the plasma membrane (29). These mechanisms likely contributed to the observed insulin dysregulation that developed in our study following dexamethasone treatment.
Glucocorticoids are often used in equine medicine for treatment of inflammatory and immune-mediated disorders (32). Importantly, treatment with glucocorticoids in horses has been associated with development of laminitis (6). Glucocorticoid-induced laminitis produces similar histological changes in the digital lamellae compared to laminitis induced by hyperinsulinemia induced with the euglycemic-hyperinsulinemic clamp (6). Therefore, insulin dysregulation induced by glucocorticoids likely has a major role in the induction of laminitis that can be observed following this treatment. In our study, treatment with dexamethasone at a common dose worsened insulin dysregulation within 7 d. Therefore, previously healthy horses treated with glucocorticoids should be considered at-risk for development of insulin dysregulation and the subsequent deleterious health side effects; this may be a particularly important consideration for those horses with other risk factors for endocrinopathic laminitis, such as obesity, breed predisposition, high-carbohydrate diet, and pituitary pars intermedia dysfunction (PPID).
An accurate diagnosis of insulin dysregulation is critical to identify horses at-risk for laminitis and other metabolic derangements associated with insulin dysregulation (hypertriglyceridemia, hyperleptinemia, and hypoadiponectinemia) to initiate appropriate treatment, such as dietary modifications and exercise (27,33). This study investigated the agreement between commonly used tests to diagnose insulin dysregulation, with moderate agreement between a basal insulin > 20 μIU/mL and the diagnostic criteria used to assess the OST (OST[Ins]60 > 60 μIU/mL, OST[Ins]60 > 45 μIU/mL). The agreement between the basal insulin > 20 μIU/mL and the diagnostic criteria used to assess the CGIT (CGIT[Gluc]45 return to baseline [glucose] CGIT[Ins]45 >100 μIU/mL) was poor, with only slight to no agreement, respectively. Furthermore, there was only moderate agreement between the results of the same test (basal insulin) repeated in the same horses 24 h later. These findings were consistent with a previous study that reported similar variability and poor agreement among the FSIGTT, basal insulin, OST, and CGIT (10).
Only moderate agreement was identified between 2 basal insulin tests performed 24 h apart. Insulin and glucose dynamics are affected by many factors, including meal feeding and fasting (14), sampling time (15), stress level (16), and medications (17). Therefore, it was not unexpected to have only moderate agreement for the diagnosis of insulin dysregulation between 2 sampling days using basal insulin. However, this test should be used as a screening test for insulin dysregulation in clinical practice, and a follow-up dynamic test should be performed if the basal insulin is < 20 μIU/mL but clinical suspicion for insulin dysregulation remains high. Similarly, diagnosis of insulin dysregulation based on the OST also resulted in moderate agreement with the basal insulin, indicating that the OST performed differently than basal insulin in the diagnosis of insulin dysregulation. The repeatability of the OST was good for the binary classification of insulin dysregulation; however, the absolute [insulin] varied greatly with repeated testing in the same horse (20). Furthermore, the OST has had poor repeatability and performed significantly different in fasted versus unfasted states (11). Alternatively, the basal insulin and the CGIT had only poor to no agreement in the diagnosis of insulin dysregulation at the baseline time point. The number of horses identified as insulin dysregulation based on the CGIT[Gluc]45 return to baseline blood [glucose] was greater than the number of horses identified as insulin dysregulation based on the basal insulin. Conversely, the number of horses identified as insulin dysregulation based on the CGIT [Ins]45 > 100 μIU/mL was less than those identified as insulin dysregulation from the basal insulin. As a result, the CGIT was both more and less sensitive compared to the basal insulin, depending on the diagnostic criteria utilized for the diagnosis of insulin dysregulation. Parameters calculated from the glucose curve following a CGIT had low repeatability compared to a moderate-to-high repeatability obtained from calculated insulin parameters (21). Therefore, when diagnosing insulin dysregulation based on the CGIT, using the glucose parameters alone should be done with caution.
The results of this study demonstrated a wide and variable level of agreement between commonly used tests for the diagnosis of insulin dysregulation. Based on these findings, performing multiple tests, in addition to insulin sensitivity proxy measurements, may be useful for a more consistent and accurate diagnosis of insulin dysregulation, particularly for monitoring response to treatment over time. The basal insulin is easy to perform and can be paired with calculation of insulin sensitivity proxy measurements and the OST or the CGIT on the same testing day. Use of the OST allows for evaluation of the enteroinsular axis following administration of an enteral carbohydrate load, which may better represent a horse’s natural response to a diet high in non-structural carbohydrates (18,19). Alternatively, the CGIT provides a better assessment of peripheral tissue insulin sensitivity (19). The insulin tolerance test (ITT) could also be performed as an alternative to the CGIT for the assessment of tissue insulin sensitivity, which would preclude the need for an insulin measurement (1). Therefore, it may be beneficial to perform both the OST and CGIT (or ITT) in a horse with suspected insulin dysregulation that was not diagnosed based on the basal insulin, as these tests evaluate different components to the pathogenesis of insulin dysregulation, resulting in a higher likelihood of an accurate diagnosis and insight as to the underlying cause.
A limitation of this study was a relatively small sample size. Furthermore, 1 horse was identified as an outlier and removed from statistical analysis of the basal insulin and glucose concentrations, MIRG, and IG, further decreasing sample size. Dexamethasone was administered orally, which may have resulted in inconsistent drug administration over time. In addition, the ELISA used to measure the insulin concentrations did not have specific cut-off values determined. However, the insulin concentration cut-off values used in this study were determined from the previously used, albeit no longer available, radioimmunoassay (RIA) (12,27,34). At insulin concentrations measured < 175 μIU/mL the RIA and ELISA had good agreement (35). We did not perform a gold-standard test for the diagnosis of insulin dysregulation, such as the FSIGTT or the EHC technique. As a result, we were unable to assess the sensitivity or specificity of the performance of the basal insulin, OST, or CGIT in the diagnosis of insulin dysregulation. However, we assessed more commonly used diagnostic tests in clinical practice and demonstrated a high degree of variability in their results, which emphasized the possibility of making an inconsistent, and potentially inaccurate, diagnosis regarding the presence or absence of insulin dysregulation if only performing 1 test.
In conclusion, dexamethasone administered at 0.08 mg/kg, PO, q24h for 7 d can induce insulin dysregulation based on insulin sensitivity proxy measurements and basal insulin, OST, and CGIT. Therefore, horses treated with glucocorticoids should be considered at risk for development of insulin dysregulation, and appropriate precautions should be implemented during the treatment period. In addition, these results demonstrated that common diagnostic tests used to diagnose insulin dysregulation have variable agreement, ranging from none to moderate; performing multiple tests may support a more accurate diagnosis of insulin dysregulation. Furthermore, if the clinician suspects insulin dysregulation based on the patient’s clinical signs and/or risk factors, then treatment strategies should be instituted, regardless of test results, as there could be a high likelihood of misdiagnosis depending on the diagnostic test(s) performed. Additional studies are required to determine more appropriate testing strategies for the diagnosis of insulin dysregulation, including which test or combination of tests should be performed and to determine more appropriate cut-off values for the diagnosis of insulin dysregulation.
Acknowledgments
Funding for this study was generously provided by the Grayson Jockey Club Research Foundation. The authors extend special thanks to the staff of the OSU Galbreath Equine Center for their excellent care of the horses. Portions of these data were presented at the ACVIM Forum On Demand, June 2020. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
Funding was provided by the Grayson Jockey Club Research Foundation.
References
- 1.Frank N, Tadros EM. Insulin dysregulation. Equine Vet J. 2014;46:103–112. doi: 10.1111/evj.12169. [DOI] [PubMed] [Google Scholar]
- 2.Asplin KE, Sillence MN, Pollitt CC, McGowan CM. Induction of laminitis by prolonged hyperinsulinaemia in clinically normal ponies. Vet J. 2007;174:530–535. doi: 10.1016/j.tvjl.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 3.de Laat MA, McGowan CM, Sillence MN, Pollitt CC. Equine laminitis: Induced by 48 h hyperinsulinaemia in Standardbred horses. Equine Vet J. 2010;42:129–135. doi: 10.2746/042516409X475779. [DOI] [PubMed] [Google Scholar]
- 4.Haffner JC, Eiler H, Hoffman RM, Fecteau KA, Oliver JW. Effect of a single dose of dexamethasone on glucose homeostasis in healthy horses by using the combined intravenous glucose and insulin test. J Anim Sci. 2009;87:131–135. doi: 10.2527/jas.2008-1179. [DOI] [PubMed] [Google Scholar]
- 5.Tiley HA, Geor RJ, McCutcheon LJ. Effects of dexamethasone on glucose dynamics and insulin sensitivity in healthy horses. Am J Vet Res. 2007;68:753–759. doi: 10.2460/ajvr.68.7.753. [DOI] [PubMed] [Google Scholar]
- 6.Johnson PJ, Slight SH, Ganjam VK, Kreeger JM. Glucocorticoids and laminitis in the horse. Vet Clin North Am Equine Pract. 2002;18:219–236. doi: 10.1016/s0749-0739(02)00015-9. [DOI] [PubMed] [Google Scholar]
- 7.Ryu SH, Kim BS, Lee CW, Yoon J, Lee YL. Glucocorticoid-induced laminitis with hepatopathy in a Thoroughbred filly. J Vet Sci. 2004;5:271–274. [PubMed] [Google Scholar]
- 8.Eustace RA, Redden RR. Iatrogenic laminitis. Vet Rec. 1990;126:586. [PubMed] [Google Scholar]
- 9.Humber KA, Beech J, Cudd TA, Palmer JE, Gardner SY, Sommer MM. Azathioprine for treatment of immune-mediated thrombocytopenia in two horses. J Am Vet Med Assoc. 1991;199:591–594. [PubMed] [Google Scholar]
- 10.Dunbar LK, Mielnicki KA, Dembek KA, Toribio RE, Burns TA. Evaluation of four diagnostic tests for insulin dysregulation in adult light-breed horses. J Vet Intern Med. 2016;30:885–891. doi: 10.1111/jvim.13934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knowles EJ, Harris PA, Elliott J, Menzies-Gow NJ. Use of the oral sugar test in ponies when performed with or without prior fasting. Equine Vet J. 2017;49:519–524. doi: 10.1111/evj.12607. [DOI] [PubMed] [Google Scholar]
- 12.Eiler H, Frank N, Andrews FM, Oliver JW, Fecteau KA. Physiologic assessment of blood glucose homeostasis via combined intravenous glucose and insulin testing in horses. Am J Vet Res. 2005;66:1598–1604. doi: 10.2460/ajvr.2005.66.1598. [DOI] [PubMed] [Google Scholar]
- 13.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: Advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 14.Bertin FR, Taylor SD, Bianco AW, Sojka-Kritchevsky JE. The effect of fasting duration on baseline blood glucose concentration, blood insulin concentration, glucose/insulin ratio, oral sugar test, and insulin response test results in horses. J Vet Intern Med. 2016;30:1726–1731. doi: 10.1111/jvim.14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noble GK, Sillence MN. Diurnal rhythm and effects of feeding, exercise and recombinant equine growth hormone on serum insulin concentrations in the horse. Equine Vet J. 2013;45:745–750. doi: 10.1111/evj.12057. [DOI] [PubMed] [Google Scholar]
- 16.Forhead AJ, Dobson H. Plasma glucose and cortisol responses to exogenous insulin in fasted donkeys. Res Vet Sci. 1997;62:265–269. doi: 10.1016/s0034-5288(97)90202-2. [DOI] [PubMed] [Google Scholar]
- 17.Kritchevsky JE, Muir GS, Leschke DHZ, Hodgson JK, Hess EK, Bertin FR. Blood glucose and insulin concentrations after alpha-2-agonists administration in horses with and without insulin dysregulation. J Vet Intern Med. 2020;34:902–908. doi: 10.1111/jvim.15747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Laat MA, McGree JM, Sillence MN. Equine hyperinsulinemia: Investigation of the enteroinsular axis during insulin dysregulation. Am J Physiol Endocrinol Metab. 2016;310:E61–72. doi: 10.1152/ajpendo.00362.2015. [DOI] [PubMed] [Google Scholar]
- 19.Bertin FR, de Laat MA. The diagnosis of equine insulin dysregulation. Equine Vet J. 2017;49:570–576. doi: 10.1111/evj.12703. [DOI] [PubMed] [Google Scholar]
- 20.Frank N, Walsh DM. Repeatability of oral sugar test results, glucagonlike peptide-1 measurements, and serum high-molecular-weight adiponectin concentrations in horses. J Vet Intern Med. 2017;31:1178–1187. doi: 10.1111/jvim.14725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bröjer J, Lindåse S, Hedenskog J, Alvarsson K, Nostell K. Repeatability of the combined glucose-insulin tolerance test and the effect of a stressor before testing in horses of 2 breeds. J Vet Intern Med. 2013;27:1543–1550. doi: 10.1111/jvim.12172. [DOI] [PubMed] [Google Scholar]
- 22.Schuver A, Frank N, Chameroy KA, Elliott SB. Assessment of insulin and glucose dynamics by using an oral sugar test in horses. J Eq Vet Sci. 2014;34:465–470. [Google Scholar]
- 23.Hackett ES, McCue PM. Evaluation of a veterinary glucometer for use in horses. J Vet Intern Med. 2010;24:617–621. doi: 10.1111/j.1939-1676.2010.0481.x. [DOI] [PubMed] [Google Scholar]
- 24.Rings LM, Swink JM, Dunbar LK, Burns TA, Toribio RE. Enteroinsular axis response to carbohydrates and fasting in healthy newborn foals. J Vet Intern Med. 2019;33:2752–2764. doi: 10.1111/jvim.15641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motulsky HJ, Brown RE. Detecting outliers when fitting data with nonlinear regression — A new method based on robust nonlinear regression and the false discovery rate. BMC Bioinformatics. 2006;7:123. doi: 10.1186/1471-2105-7-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meier AD, de Laat MA, Reiche DB, et al. The oral glucose test predicts laminitis risk in ponies fed a diet high in nonstructural carbohydrates. Domest Anim Endocrinol. 2018;63:1–9. doi: 10.1016/j.domaniend.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Durham AE, Frank N, McGowan CM, et al. ECEIM consensus statement on equine metabolic syndrome. J Vet Intern Med. 2019;33:335–349. doi: 10.1111/jvim.15423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frank N. Equine metabolic syndrome. Vet Clin North Am Equine Pract. 2011;27:73–92. doi: 10.1016/j.cveq.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Beaupere C, Liboz A, Fève B, Blondeau B, Guillemain G. Molecular mechanisms of glucocorticoid-induced insulin resistance. Int J Mol Sci. 2021:22. doi: 10.3390/ijms22020623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510:84–91. doi: 10.1038/nature13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakoda H, Ogihara T, Anai M, et al. Dexamethasone-induced insulin resistance in 3T3-L1 adipocytes is due to inhibition of glucose transport rather than insulin signal transduction. Diabetes. 2000;49:1700–1708. doi: 10.2337/diabetes.49.10.1700. [DOI] [PubMed] [Google Scholar]
- 32.Leclere M. Corticosteroids and immune suppressive therapies in horses. Vet Clin North Am Equine Pract. 2017;33:17–27. doi: 10.1016/j.cveq.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Sparks JD, Sparks CE, Adeli K. Selective hepatic insulin resistance, VLDL overproduction, and hypertriglyceridemia. Arterioscler Thromb Vasc Biol. 2012;32:2104–2112. doi: 10.1161/ATVBAHA.111.241463. [DOI] [PubMed] [Google Scholar]
- 34.Treiber KH, Kronfeld DS, Hess TM, Byrd BM, Splan RK, Staniar WB. Evaluation of genetic and metabolic predispositions and nutritional risk factors for pasture-associated laminitis in ponies. J Am Vet Med Assoc. 2006;228:1538–1545. doi: 10.2460/javma.228.10.1538. [DOI] [PubMed] [Google Scholar]
- 35.Borer-Weir KE, Bailey SR, Menzies-Gow NJ, Harris PA, Elliott J. Evaluation of a commercially available radioimmunoassay and species-specific ELISAs for measurement of high concentrations of insulin in equine serum. Am J Vet Res. 2012;73:1596–1602. doi: 10.2460/ajvr.73.10.1596. [DOI] [PubMed] [Google Scholar]