Abstract
Background
In the thoughts of all orthopedicians, the emergence of drug-resistant and biofilm-forming bacterial infections at orthopedic surgical sites is the most feared problem. Thus, this study aimed to determine the bacteriological profiles, antimicrobial susceptibility patterns, and biofilm forming ability of isolates, as well as factors associated with orthopedic surgical site infections (OSSIs).
Methods
An institution-based cross-sectional study was conducted from March 1st, 2021, to February 30th, 2022 at Arba Minch General Hospital. About 245 suspected orthopedic patients with surgical site infection were enrolled and structured questionnaires were used to collect the required information. Wound swabs or pus aspirates were aseptically collected. The frequency and type of bacterial pathogen(s), antimicrobial susceptibility pattern, and biofilm formation were used to determine and characterize the magnitude of OSSIs. SPSS version 25 was used to analyze factors associated with OSSIs.
Results
The overall magnitude of symptomatic OSSIs was 29.4% (72/245). External fixation [AOR = 4.761, 95% CI: (1.108–20.457)], implant use [AOR = 3.470, 95% CI: (1.460–8.246)], length of time for surgery [AOR = 3.225, 95% CI: (1.545–6.731)], and post-operative hospitalization [AOR = 4.099, 95% CI: (2.026–8.293)] were all statistically significant. Staphylococcus aureus was the most frequently isolated bacteria, accounting for 76%. Methicillin-resistant was observed in 57.9% and 40% of isolated S. aureus (MRSA) and coagulase-negative staphylococci (CoNS), respectively. One-third of the isolated E. faecium was vancomycin-resistant (VRE). Overall, 67.1% (51/76) of isolates were multidrug-resistant (MDR). About 27.6% (21/76) of isolates were found to be strong biofilm producers.
Conclusion
OSSIs were shown to be caused by a significant number of drug-resistant and biofilm-producing bacterial isolates. To mitigate the problem, aseptic surgical practice and conventional wound management, as well as constant observation of antimicrobial resistant patterns, should be followed.
Keywords: antimicrobial susceptibility, bacterial pathogen, biofilm, orthopedic surgical site infection, Arba Minch, Ethiopia
Introduction
Surgical site infection (SSI) following orthopedic surgery is one of the most common complications, and it remains a serious global issue for patients who have had any type of orthopedic surgery. 1–3 Surgical site infection (SSI) is a major problem in orthopedics as it is difficult to rid the infection of the bone and joint and is associated with lifelong recurrence risks of around 10–20%.4 Despite advances in infection control, aseptic surgical techniques, and the use of antibiotic prophylaxis, the global prevalence of orthopedic SSI is estimated to be between 1.4 and 41.9%.2,5 Orthopedic surgical site infection (OSSI) is defined as an infection that occurs within 30 days following a surgical procedure or within one year if the surgical site is repaired with an implant.5–8
SSIs in orthopedic surgery have a variety of clinical, financial, and social consequences, including longer hospital stays, increased morbidity and mortality, increased risk of readmission and revision surgery (debridement), higher treatment costs, and compromised health outcomes.6,9 The presence of either too many replicating microbes (≥ 105 CFU/gram of tissue) or too many species in the wound base has a detrimental effect on the development of infection and delays wound healing.10 The infection can manifest as superficial incisions, deep incisions, bone infections, or infections involving a newly-implanted prosthetic device, resulting from an adverse reaction to an infectious agent or its toxin.1,11 Although the distribution of many microorganisms is affected by a variety of epidemiological factors, Staphylococcus aureus, Coagulase Negative Staphylococcus spp., Pseudomonas aeruginosa, Enterococci spp., Acinetobacter spp., Klebsiella spp, Escherichia coli, and Proteus spp. are the most commonly reported bacterial pathogens.5,11,12
The sources of the pathogens can be either endogenous or exogenous, but most of the orthopedic SSIs arise from the endogenous bacterial flora under certain favorable conditions.11,13,14 Several factors influence orthopedic surgery wound healing and determine the likelihood for infection, in addition to the level of microbial contamination at an incision site, which has been a major issue.6,13,15,16 These include host factors (such as dietary state, lifestyle, comorbidities, immune status, and concurrent infections), and pre-, intra-, and post-operative procedural-related variables, as well as the surgeon’s ability or performance.1,4,16 However, some variables are not modifiable, such as age and gender, while other potential factors can be improved to increase the likelihood of a successful surgical outcome.1,6
Furthermore, implant-related infections in orthopedic surgery continue to be a challenge for orthopedic surgeons due to several mechanisms, including corrosion, isolation from the immune response, resistance to antimicrobial therapy, and compromise of blood supply. The implant makes the adjacent tissues susceptible to both immediate and delayed infection, eventually undergoing a phenotypic alteration to develop a biofilm matrix over the implant surface.1,7,10,11,17,18 Moreover, open fracture fixation increases the risk of infection due to the exposure of the implant to the outside environment.5,19,20 After implant fixation, the risk of infection is estimated to range from 0.5% to 30% (0.5–2% in fixation of closed fractures to 30% after fixation of open fractures).5,13
The OSSI is typically managed based on the clinical manifestation (pain at a surgically created wound accompanied by erythema, swelling, local tenderness, or purulent discharge at the wound site, as well as changes in the range of motion and other deformities)2,7,17 and necessitates close collaboration between the surgeon and other supportive healthcare members.1,21 However, the emergence of multidrug-resistant and biofilm-forming bacterial strains has made post-operative orthopedic infection management and treatment a severe concern in surgical practice.14,22 Moreover, methicillin-resistant S. aureus (MRSA), vancomycin-resistant Enterococcus spp and extended-spectrum beta-lactamase (ESBL) gram-negative bacteria have a particular concern.11,23 This problem gets more complicated in developing countries due to the irrational use of broad-spectrum antibiotics and poor infection prevention programs.1,5,23 Thus, with the perpetual emergence of antibiotic-resistant strains, it is important to know the prevailing bacterial etiology and their antimicrobial-resistant patterns.8,22
Despite the fact that several studies on the prevalence of SSIs have been carried out in different areas of Ethiopia, with epidemiological variance from hospital to hospital and even from ward to ward, no independent investigation on OSSIs has yet been carried out.14,23 As a result, the goal of this study was to find out bacterial infection at the orthopedic surgical site, antimicrobial susceptibility patterns, and biofilm detection of isolates, and associated factors among patients undergoing orthopedic surgery at Arba Minch General Hospital, Southern Ethiopia.
Materials and Methods
Study Area, and Period
The study was conducted at the orthopedic ward of Arba Minch General Hospital, Southern Ethiopia from 20th February 2021 to 30th January 2022. The Hospital is found in Arba Minch town of Gamo Zone Southern Nations, Nationalities’ and Peoples’ Region (SNNPR), Southern Ethiopia. Arba Minch is the seat of administration of the Gamo zone which is located 454 kms away from the capital city of Ethiopia (Addis Ababa). Arba Minch General Hospital was established in 1961 E.C during Empierer Hailesilasie and provides preventive, curative, and rehabilitative care for more than 1.5 million people from the Gamo zone and other nearby zones. Though the department has been launched recently, orthopedic surgery is one of the services given in the hospital; however, post-surgical orthopedic infection was a challenging problem as we have gotten information from the department (orthopedics).
Study Design, Population, and Eligibility Criteria
An institution-based cross-sectional study design was conducted. All clinically suspected patients with post-surgical orthopedic infection admitted to the ward, as well as those who had followed up within 30 days after surgery or one year if an implant was in place, were included.5 Patients were excluded if they (a) showed signs and symptoms of infection within the first 48 hours of surgery, (b) had undergone orthopedic surgery at another hospital, or (c) were unable to respond or severely ill.
Sample Size Determination and Sampling Technique
The required sample size was calculated based on the following assumptions: P-value = 82.4% obtained from a previous study at Tikur Anbessa Specialized Hospital in Addis Ababa, Ethiopia;14 95% confidence level (Zα/2= 1.96), and 5% margin of error. The total sample size was 245 after accounting for the 10% non-response rate. The overall sample size was determined using a consecutive sampling technique.
Operational Definition
Orthopedic Surgical Site Infection
If there is a significant growth of pathogenic bacteria after overnight incubation of surgical wound swab samples within appropriate culture media.
Gustilo and Anderson’s Fracture Wound Grading System
Grade I: a clean, 1 cm long wound with no significant soft-tissue damage. The skin wound is usually caused by the puncture of a bone fragment from within. The skin wound is usually caused by the puncture of a bone fragment from within. Grade II: wound >1 cm; moderate soft-tissue damage, minimal periosteal stripping. Grade III-A: extensive soft-tissue destruction with adequate osseous coverage. Grade III-B: extensive soft-tissue destruction with periosteal stripping, which requires a vascularized tissue transfer for soft-tissue coverage. Grade III-C: extensive soft-tissue destruction with neurovascular injury that requires repair.10,19
Multidrug-Resistant (MDR)
Isolates that resist at least one antimicrobial drug in three or more antimicrobial categories.24
Data Collection and Processing
The primary source of data was a patient who had undergone orthopedic surgery and was clinically suspected of having a surgical site infection. When necessary, the patient’s medical record and the responsible surgeon were considered. A structured questionnaire administered through a face-to-face interview was used to collect patient-specific socio-demographic characteristics, medical-surgical profiles, clinical conditions, and behavioral factors.
Sample Collection and Transportation
Orthopedicians collected wound swabs or pus aspirate samples aseptically from the wounds of consented patients for microbiological examination. The wound swab samples were taken by rotating a sterile cotton-tipped applicator over a 1 cm2 region for 5 seconds with enough pressure to expel fluid and bacteria from the wound tissue to the surface. The applicators were put deep into the wounds to avoid pollutants that are generally located on the surface of the wounds. The samples were then placed in a sterile test tube containing nutrient broth or Amies transport medium (Oxoid Ltd, UK) and processed within 1 hour of collection in the Medical Microbiology and Parasitology laboratory of Arba Minch University’s College of Medicine and Health Sciences; if not processed within 1 hour, the samples were kept in a 4°C refrigerator until analysis.25
Culturing and Bacterial Identification
Standard microbiological protocols were used to inoculate samples onto 5% sheep blood agar medium, mannitol salt agar, and MacConkey agar. The plates were aerobically incubated for 24–72 hours at 37°C, and one medium per sample was incubated within a candle jar to create a 5–10% CO2 concentration for micro-aerophilic bacteria. All positive cultures were processed for the identification of isolates, drug susceptibility tests, and biofilm detection. Identifications were performed by their colonies’ characteristic appearance on the respective media, gram staining reaction, and confirmed by the pattern of biochemical reactions as described in Bergey’s Manual of Systemic Bacteriology.26
Antimicrobial Susceptibility Testing
An antimicrobial susceptibility test was performed for each pure isolate of bacteria on Mueller Hinton agar by using Kirby-Bauer disk diffusion techniques.27 Accordingly, detailed Clinical and Laboratory Standards Institute (CLSI) guidelines.25 For each category of gram-positive and gram-negative bacteria isolates were tested against, amoxicillin (30 µg, BD), amoxycillin-clavulanic acid (30 µg, BD), ceftazidime (30 µg, BD), cefotaxime (30 µg, BD), ceftriaxone (30 µg, BD), chloramphenicol (30 µg, BD), clindamycin (2 µg, BD), gentamicin (10 µg, BD), cotrimoxazole (25 μg), tetracycline (30 µg, BD), ciprofloxacin (5 µg, BD), penicillin (10 units, BBL), cefoxitin (30 μg), erythromycin (15 µg, BD), piperacillin (100 μg), cefepime (30μg), streptomycin (10 μg) and meropenem (10 μg). Then after overnight incubation at 37°C, the diameter of zone of inhibition was measured by millimetre and interpreted as sensitive, intermediate and resistant.25
Detection of Biofilm Production
The Microtiter plate (MtP) assay was used to determine in vitro biofilm formation of isolated bacteria with the following procedures:28 bacterial suspensions having a final volume of 200 µL were prepared by using 20 µL of bacterial suspension adjusted to 0.5 McFarland standards and 180µL of Trypticase Soy Broth (TSB) supplemented with 1% glucose. Then suspensions were inoculated into 96 well flat-bottomed sterile polystyrene plastic microtiter plates along with positive control organisms and incubated for 24 hours at 37 °C. The only sterile broth was used as a blank and as a negative control. After incubation time, isolates of biofilms formed on the wells of microtiter plates were tapped and stained following standard procedures [Appendix 1]. The optical density (OD) of each microplate was measured (repeatedly three times) spectrophotometrically by automated ELISA Auto-reader at a wavelength of 570 nm. The results were categorized based on the calculated cutoff optical density (ODc) value. ODc was calculated and defined as three standard deviations above the mean OD of the negative control.28
The average OD of the negative control was found to be 0.0213 (0.0213; 0.0212; 0.0215) with standard deviation (SD) of 0.0021. As a result, ODc-value was 0.0213 + 3*0.0021= 0.2193. Then the final OD value of a tested strain was determined as average OD value of the strain after subtracting ODc value (0.2193) and classified as follows: bacterial OD ≤ 0.2193 = biofilm non-former; OD > 0.2193 but < 0.4386 = weak biofilm former; OD > 0.4386 but < 0.8772 = moderate biofilm former; and if OD ≥ 0.8772= strong biofilm former as per Stepanović et al description.28
Data Quality Management
From data collection to final laboratory identification, data quality was assured by closely following standard operating procedures (SOPs) and providing proper training and follow-up for data and sample collectors. Before the actual data collection, a pre-test was conducted on 5% of the entire sample size. Throughout the data collection period, the investigator provided close supervision. The sterility of culture media was confirmed by incubating 5% of the prepared media at 35–37°C overnight. The batches of media that showed positive growth were discarded. Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 25923, and Enterococcus faecalis ATCC 29212, all obtained from the Ethiopian Public Health Institute, were used to test the prepared media and antibiotic disc performance.25,26
Data Analysis
Data were coded and entered by using Epi-data version 4.6, and then it was exported to SPSS version 25 for further cleaning and analysis. Descriptive statistics like, frequency, mean, and percentage were calculated. A binary logistic regression model was used to assess associations. The odds ratio (OR) with its corresponding 95% confidence interval (CI) was estimated. Variables with a p-value < 0.25 in bivariable analysis were considered as candidates for further multivariable analysis. The strength of the associations was measured using an adjusted odds ratio (AOR) with a 95% confidence interval, and variables with a p-value of ≤ 0.05 were considered statistically significant.
Ethical Considerations
The project was approved by the Arba Minch University College of Medicine and Health Sciences’ Institutional Research Ethics Review Board (Ref. No. IRB/1020/21).Written permission letter was also obtained from the hospital. In accordance with the Helsinki Declaration, all study participants were made aware of the study’s goal and their right to refuse or accept it. Written informed consent was obtained prior to the interview, as well as informed written assent from parents or guardians for individuals under the age of 18 years old. To combat with the COVID-19 problem, all standard precautions were taken, such as wearing personal protective equipment when collecting and processing the sample. Important laboratory results were promptly communicated to the inquiring orthopedic doctors, along with the patient’s card number.
Results
Socio-Demographic Characteristics
During the study period, 245 eligible study participants were investigated. Males made up 67.3% (165/245) of the patients suspected of having orthopedic surgery site infections. This could be explained by the fact that males are more likely than females to suffer orthopedic injuries. This is due to the fact that men work in the transportation industry, construction, farming, and day labor, and are more likely to engage in interpersonal violence. The majority of patients (44.9%) were between the ages of 19 and 38, with a mean age of 35.64 (std. 16.47) and a range of 7 to 70 years. 3.3% of orthopedic patients were illiterate, with more than half having completed secondary or higher education. About 56.7% of participants were from rural areas (Table 1).
Table 1.
Socio-Demographic Characteristics of Patients with Suspected Orthopedic Surgical Site Infection at Arba Minch General Hospital
| Variables | Categories | Frequency (n) | Percentage (%) |
|---|---|---|---|
| Gender | Male | 165 | 67.3 |
| Female | 80 | 32.7 | |
| Age (years) | ≤ 18 | 36 | 14.7 |
| 19–38 | 110 | 44.9 | |
| 39–58 | 68 | 27.8 | |
| 59–78 | 31 | 12.7 | |
| Residence | Urban | 106 | 43.3 |
| Rural | 139 | 56.7 | |
| Educational status | Illiterate | 8 | 3.3 |
| Primary | 85 | 34.7 | |
| Secondary | 124 | 50.6 | |
| Diploma & above | 28 | 11.4 | |
| Occupation | Farmer | 48 | 19.6 |
| House wife | 19 | 7.8 | |
| Merchant | 52 | 26.9 | |
| Student | 66 | 21.2 | |
| Employee | 33 | 13.5 | |
| Driver | 27 | 11.0 |
Bacterial Isolates from Patients with Suspected Orthopedic Surgical Site Infection
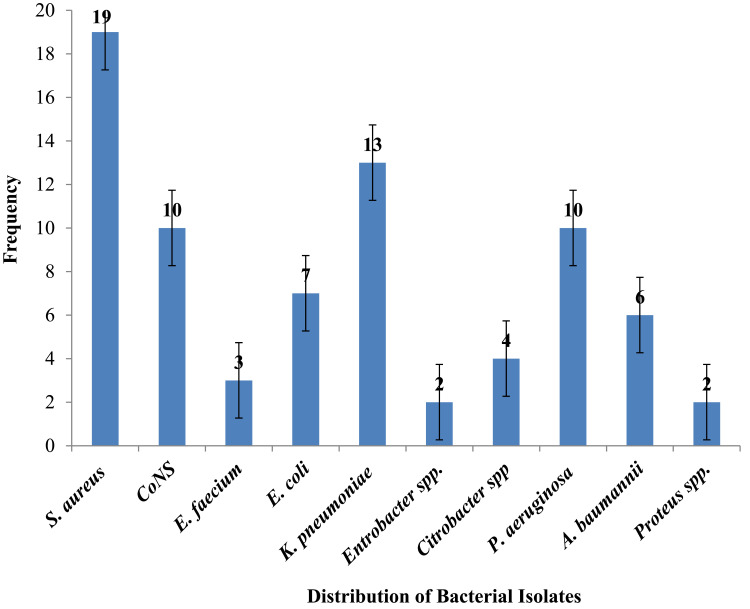
Among the 245 orthopedic surgical wound samples subjected to aerobic bacteriological culture, bacterial growth was found in 29.4% (72/245), with 5.5% (4/72) of culture positive wounds having double bacterial infections, bringing the total number of bacteria isolates to seventy-six, belonging to ten distinct genuses. The double isolated bacteria were Staphylococcus aureus and Klebsiella pneumoniae, 25% (1/4), Pseudomonas aeruginosa and K. pneumoniae, 25% (1/4), CoNS and P. aeruginosa, 25% (1/4), and E. coli and Acinetobacter baumannii, 25% (1/4). Gram–positive bacteria comprised 42.1% (32/76), whereas Gram–negative bacteria constituted 57.9% (44/76) of the total isolates, resulting in a gram-positive to gram-negative ratio of 0.727:1. S. aureus was the most frequently isolated bacteria, accounting for 25% (19/76), followed by K. pneumoniae 17.1% (13/76), CoNS 13.2% (10/76), and P. aeruginosa 13.2% (10/76) (Figure 1).
Figure 1.
Frequency of Bacterial Isolates from Infection Suspected Orthopedic Surgical Wounds at Arba Minch General Hospital.
Socio-Demographic Factors Associated with Orthopedic Surgical Site Infections
Among all the bacterial isolates, 62.5% (45/72) of the culture positives were from males, and 37.5% (27/72) were from female orthopedic patients. However, there was no significant association between gender and culture results, though the samples were more from the male population (Male: Female = 2.063:1). In terms of age, 44.4% of cultured positive orthopedic surgical wounds (32/72) were between the ages of 19 and 38 years old. 52.8% of orthopedic patients with culture positive wound results were from rural areas, whereas 54.2% of orthopedic patients with a secondary educational level had orthopedic surgical site infection. However, there was no significant association between socio-demographic characteristics and orthopedic surgical site infections (Table 2).
Table 2.
Association of Socio-Demographic Factors with Orthopedic Surgical Site Infection at Arba Minch General Hospital
| Variables | Category | Culture Result | COR (95% CI) | AOR (95% CI) | P-value | |
|---|---|---|---|---|---|---|
| Positive (%) | Negative (%) | |||||
| Sex | Male | 45 (62.5) | 120 (69.4) | 0.736 (0.414–1.310) | 0.682 (0.341–1.364) | 0.279 |
| Female | 27 (37.5) | 53 (30.6) | 1 | 1 | ||
| Age | ≥18 | 12 (16.7) | 24 (13.9) | 1.437 (0.497–4.157) | 1.689 (0.460–6.198) | 0.429 |
| 19–38 | 32 (44.4) | 78 (45.1) | 1.179 (0.478–2.912) | 1.606 (0.530–4.868) | 0.403 | |
| 39–58 | 20 (27.8) | 48 (27.7) | 1.198 (0.459–3.125) | 1.750 (0.539–5.679) | 0.351 | |
| 59–78 | 8 (11.1) | 23 (13.3) | 1 | 1 | ||
| Education | Illiterate | 1 (1.4) | 7 (4.0) | 2.597 (0.303–22.276) | ||
| Primary | 23 (31.9) | 62 (35.8) | 3.212 (0.382–27.008) | |||
| Secondary | 39 (54.2) | 85 (49.1) | 3.316 (0.353–31.158) | |||
| College & above | 9 (12.5) | 19 (11.0) | 1 | |||
| Residence | Urban | 34 (47.2) | 72 (41.6) | 1 | 1 | |
| Rural | 38 (52.8) | 101 (58.4) | 1.255 (0.722–2.181) | 0.664 (0.341–1.293) | 0.228 | |
| Occupation | Farmer | 10 (13.9) | 38 (22.0) | 1 | 1 | |
| Housewife | 6 (8.3) | 13 (7.5) | 1.754 (0.532–5.777) | 0.356 | ||
| Student | 19 (26.4) | 47 (27.2) | 1.536 (0.639–3.693) | 0.337 | ||
| Merchant | 16 (22.2) | 36 (20.8) | 1.689 (0.678–4.205) | 0.260 | ||
| Employee | 13 (18.1) | 20 (11.6) | 2.470 (0.921–6.623) | 0.072 | ||
| Driver | 8 (11.1) | 19 (11.0) | 1.600 (0.543–4.714) | 0.394 | ||
Abbreviations: COR, crude odds ratio; AOR, adjusted odds ratio; CL, confidence interval.
Clinical and Behavioral Factors Associated with Orthopedic Surgical Site Infections
A total of 89.4% of study participants suspected of having orthopedic surgical site infections were admitted to the hospital for more than 48 hours, of which 27.9% of patients had OSSIs. Orthopedic patients who had just undergone external fixation (35.1%), open reduction and internal fixation (44.4%), and fusion (8.9%) had a culture-positive orthopedic surgical site infection of 55.6%, 31.9%, and 5.6%, respectively. The overall orthopedic surgery with the use of implants in this study was 74.7%, of which 33.3% of patients had OSSIs. Of the length of time for surgery, about 24.5% of the procedures were longer than 2 hours, with an infection rate of 43.3%. As per Gustilo and Anderson’s classification, the majority (44.1%) of patients suspected of orthopedic surgical site infection were present in the class II wound category, with an infection rate of 27.8%. Orthopedic patients with underlying disease and cigarette-addicted had an orthopedic surgical site infection rate of 50% and 9.7%, respectively (Table 3).
Table 3.
Association of Clinical and Behavioral Factors with Orthopedic Surgical Site Infections at Arba Minch General Hospital
| Variables | Category | Culture-Result | COR (95% CI) | AOR (95% CI) | P-value | |
|---|---|---|---|---|---|---|
| Positive (%) | Negative (%) | |||||
| Patient type | Admitted | 61 (27.9%) | 158 (72.1%) | 0.526 (0.229–1.210) | ||
| Follow up | 11 (42.3%) | 15 (57.7%) | 1 | |||
| Site of fracture | Upper extremity | 17 (31.5%) | 37 (68.5%) | 0.613 (0.184–2.043) | ||
| Lower Extremity | 49 (27.7%) | 128 (72.3%) | 0.510 (0.168–1.547) | |||
| Other | 6 (42.9%) | 8 (57.1%) | 1 | |||
| Nature of Surgery | Emergency | 48 (26.8%) | 131 (73.2%) | 0.641 (0.352–1.169) | ||
| Elective | 24 (36.4%) | 42 (63.6%) | 1 | |||
| Prophylaxis use before 30 mint of surgery | Yes | 70 (29.3%) | 169 (70.7%) | 0.828 (0.148–4.627) | ||
| No | 2 (33.3%) | 4 (66.7%) | 1 | |||
| Type of surgery | External fixation | 45 (52.3%) | 41 (47.7%) | 5.122 (1.373–19.112) | 4.761 (1.108–20.457) | 0.036 |
| Open Reduction and Internal fixation | 18 (17.3%) | 86 (82.7%) | 0.977 (0.254–3.754) | 0.959 (0.228–4.376) | 0.955 | |
| Fusion | 4 (18.2%) | 18 (81.8%) | 1.037 (0.199–5.410) | 1.319 (0.221–7.881) | 0.761 | |
| Amputation | 1 (14.3%) | 6 (85.7%) | 0.778 (0.067–9.076) | 1.420 (0.105–19.203) | 0.792 | |
| Hemiarthroplasty | 1 (11.1%) | 8 (88.9%) | 0.583 (0.052–6.587) | 0.361 (0.027–4.861) | 0.443 | |
| Repair | 3 (17.6%) | 14 (82.4%) | 1 | |||
| Use of implant | Yes | 61 (33.3%) | 122 (66.7%) | 2.318 (1.128–4.765) | 3.470 (1.460–8.246) | 0.005 |
| No | 11 (17.7%) | 51 (82.3%) | 1 | 1 | ||
| Length of time for surgery | <2hrs | 46 (24.9%) | 139 (75.1%) | 1 | 1 | |
| 2–4hrs | 26 (43.3%) | 34 (56.7%) | 2.311 (1.256–4.252) | 3.225 (1.545–6.731) | 0.002 | |
| Post-operative hospitalization | <7days | 38 (21.0%) | 143 (79.0%) | 1 | ||
| ≥7days | 34 (53.1%) | 30 (46.9%) | 4.265 (2.323–7.829) | 4.099 (2.026–8.293) | 0.001 | |
| Wound type | Class I | 9 (14.3%) | 54 (85.7%) | 1 | 1 | |
| Class II | 30 (27.8%) | 78 (72.2%) | 2.308 (1.015–5.249) | 1.390 (0.527–3.666) | 0.506 | |
| Class III A | 12 (42.9%) | 16 (57.1%) | 4.500 (1.609–12.588) | 2.090 (0.645–6.773) | 0.219 | |
| Class III B | 11 (44.0%) | 14 (56.0%) | 4.714 (1.635–13.594) | 2.490 (0.711–8.720) | 0.154 | |
| Class III C | 10 (47.6%) | 11 (52.4%) | 5.455 (1.798–16.545) | 2.738 (0.740–10.131) | 0.131 | |
| Comorbidity* | Yes | 5 (33.3%) | 10 (66.7%) | 2.507 (0.703–8.942) | ||
| No | 67 (29.1%) | 163 (70.9%) | 1 | |||
| Habit of Smoking | Yes | 7 (9.7%) | 26 (15.0%) | 0.609 (0.251–1.476) | ||
| No | 65 (90.3%) | 147 (85.0%) | 1 | |||
Notes: *Human Immunodeficiency Virus (HIV) (n=4), Diabetic Mellitus (DM) (n=5), Hepatitis B virus (HBV) (n=3), Hypertension (n=3).
Abbreviations: COR, crude odds ratio; AOR, adjusted odds ratio; CL, confidence interval.
Multivariable logistic regression analysis revealed that orthopedic patients who underwent external fixation [AOR = 4.761, 95% CI: (1.108–20.457)], orthopedic procedures with implants [AOR = 3.470, 95% CI: (1.460–8.246)], orthopedic surgical procedures lasting more than two hours than procedures lasting less than two hours [AOR = 3.225, 95% CI: (1.545–6.731)], and post-operative hospitalized for more than or equal to seven days [AOR = 4.099, 95% CI: (2.026–8.293)] were all found to be statistically significant with OSSIS (Table 3).
Antimicrobial Susceptibility Patterns of Bacterial Isolates
Among Gram-positive bacterial isolates, resistance was observed against penicillin (75%), erythromycin (53.1%), cotrimoxazole (50%), gentamycin (43.8%), and clindamycin (43.8%). The predominant isolate, S. aureus, showed 78.9%, 63.2%, and 57.9% resistance to penicillin, cotrimoxazole, and erythromycin, respectively. 57.9% (11/19) of isolated S. aureus were methicillin-resistant (MRSA). Likewise, 40% (4/10) of isolated CoNS were methicillin-resistant (MR-CoNS). More than 66.7% of isolated E. faecium were resistant to penicillin and erythromycin. Similarly, one-third of isolated E. faecium was resistant to vancomycin (VRE), while 66.7% of isolates were susceptible to chloramphenicol and vancomycin (Table 4).
Table 4.
Antimicrobial-Resistant Pattern of Gram-Positive Bacterial Isolates from Infection Suspected Orthopedic Surgical Wounds at Arba Minch General Hospital
| Drugs | Pattern | S. aureus (n=19) | CoNS (n=10) | E. faecium (n=3) | Total (n=32) |
|---|---|---|---|---|---|
| No (%) | No (%) | No (%) | No (%) | ||
| PEN | S | 3(15.8) | 2(20) | 0(0) | 5(15.6) |
| I | 1(5.3) | 2(20) | 0(0) | 3(9.4) | |
| R | 15(78.9) | 6(60) | 3(100) | 24(75) | |
| CXT | S | 8(42.1) | 6(60) | NA | 14(43.8) |
| I | 0(0) | 0(0) | NA | 0(0) | |
| R | 11(57.9) | 4(40) | NA | 15(46.9) | |
| GEN | S | 8(42.1) | 6(60) | NA | 14(43.8) |
| I | 1(5.3) | 0(0) | NA | 1(3.1) | |
| R | 10(52.6) | 4(40) | NA | 14(43.8) | |
| ERY | S | 6(31.6) | 5(50) | 1(33.3) | 12(37.5) |
| I | 2(10.5) | 1(10) | 0(0) | 3(9.4) | |
| R | 11(57.9) | 4(40) | 2(66.7) | 17(53.1) | |
| TET | S | 7(36.8) | 4(40) | 0(0) | 11(34.4) |
| I | 3(15.8) | 2(20) | 2(66.7) | 7(21.9) | |
| R | 9(47.3) | 4(40) | 1(33.3) | 14(43.8) | |
| DOX | S | 8(42.1) | 5(50) | 1(33.3) | 14(43.8) |
| I | 2(10.5) | 1(10) | 1(33.3) | 4(12.5) | |
| R | 9(47.3) | 4(40) | 1(33.3) | 14(43.8) | |
| CLD | S | 6(31.6) | 5(50) | NA | 11(34.4) |
| I | 3(15.8) | 1(10) | NA | 4(12.5) | |
| R | 10(52.6) | 4(40) | NA | 14(43.8) | |
| COT | S | 5(26.3) | 6(60) | NA | 11(34.4) |
| I | 2(10.5) | 0(0) | NA | 2(6.3) | |
| R | 12(63.2) | 4(40) | NA | 16(50) | |
| VAN | S | NA | NA | 2(66.7) | 2(6.3) |
| I | NA | NA | 0(0) | 0(0) | |
| R | NA | NA | 1(33.3) | 1(3.1) | |
| CHL | S | 8(42.1) | 6(60) | 2(66.7) | 16(50) |
| I | 3(15.8) | 1(10) | 0(0) | 4(12.5) | |
| R | 8(42.1) | 3(30) | 1(33.3) | 12(37.5) |
Abbreviations: PEN, Penicillin; CXT, Cefoxitin; GEN, Gentamicin; ERY, Erythromycin; TET, Tetracycline; DOX, Doxycycline; CLD, Clindamycin; COT, Cotrimoxazole; VAN, Vancomycin; CHL, Chloramphenicol; NA, not applicable based on CLSI 2020 guideline recommendation; S, sensitive; I, intermediate; R, resistant; CoNS, Coagulase Negative Staphylococci; spp, species.
More than 43.2% of Gram-negative bacterial isolates were resistant to ampicillin, cefepime, ceftriaxone, cefuroxime, tetracycline, and ciprofloxacin, while 41%, 47.7%, and 63.6% of isolates showed better response to augmentin, gentamicin, and meropenem, respectively. Ampicillin, cefepime, cefoxitin, ceftriaxone, cefuroxime, and ciprofloxacin resistant were found in 69.2% of K. pneumoniae isolates. Piperacillin and meropenem susceptibility was found in 60% of P. aeruginosa isolates, but ceftazidime resistant was found in 70%. Cefepime, ceftazidime, and ciprofloxacin resistant were found in 66.7% of the A. baumannii isolates, whereas isolated Proteus spp. were found to be susceptible to augmentin, cefoxitin, gentamicin, meropenem, and chloramphenicol (Table 5).
Table 5.
Antimicrobial Resistant Pattern of Gram-Negative Bacterial Isolates from Infection Suspected Orthopedic Surgical Wounds at Arba Minch General Hospital
| Drugs | Pattern | K. pneumoniae (n=13) | E. coli (n=7) | P. aeruginosa (n=10) | Citrobacter spp. (n=4) | Entrobacter spp. (n=2) | A. baumannii (n=6) | Proteus spp. (n=2) | Total (n=44) |
|---|---|---|---|---|---|---|---|---|---|
| No (%) | No (%) | No (%) | No (%) | No (%) | No (%) | No (%) | No (%) | ||
| AMP | S | 3(23.1) | 2(28.6) | NA | 1(25) | 1(50) | NA | 0(0) | 7(15.9) |
| I | 1(7.7) | 1(14.3) | NA | 0(0) | 0(0) | NA | 0(0) | 2(4.5) | |
| R | 9(69.2) | 4(57.1) | NA | 3(75) | 1(50) | NA | 2(100) | 19(43.2) | |
| PIP | S | NA | NA | 6(60) | NA | NA | 3(50) | NA | 9(20.5) |
| I | NA | NA | 1(10) | NA | NA | 0(0) | NA | 1(2.3) | |
| R | NA | NA | 3(30) | NA | NA | 3(50) | NA | 6(13.6) | |
| AUG | S | 7(53.8) | 4(57.1) | NA | 3(75) | 2(100) | NA | 2(100) | 18(41) |
| I | 1(7.7) | 1(14.3) | NA | 0(0) | 0(0) | NA | 0(0) | 2(4.5) | |
| R | 5(38.5) | 2(28.6) | NA | 1(25) | 0(0) | NA | 0(0) | 8(18.2) | |
| CFP | S | 2(15.4) | 1(14.3) | 2(20) | 1(25) | 0(0) | 2(33.3) | 0(0) | 8(18.2) |
| I | 2(15.4) | 1(14.3) | 1(10) | 0(0) | 1(50) | 0(0) | 2(100) | 7(15.9) | |
| R | 9(69.2) | 5(71.4) | 7(70) | 3(75) | 1(50) | 4(66.7) | 0(0) | 29(65.9) | |
| CXT | S | 4(30.8) | 3(42.9) | NA | 2(50) | 1(50) | NA | 2(100) | 12(27.3) |
| I | 0(0) | 0(0) | NA | 0(0) | 0(0) | NA | 0(0) | 0(0) | |
| R | 9(69.2) | 4(57.1) | NA | 2(50) | 1(50) | NA | 0(0) | 16(36.4) | |
| CTR | S | 3(23.1) | 2(28.6) | NA | 1(25) | 0(0) | 2(33.3) | 1(50) | 9(20.5) |
| I | 1(7.7) | 1(14.3) | NA | 1(25) | 1(50) | 1(16.7) | 0(0) | 5(11.4) | |
| R | 9(69.2) | 4(57.1) | NA | 2(50) | 1(50) | 3(50) | 1(50) | 20(45.5) | |
| CRX | S | 2(15.4) | 1(14.3) | NA | 1(25) | 1(50) | NA | 0(0) | 5(11.4) |
| I | 2(15.4) | 1(14.3) | NA | 0(0) | 0(0) | NA | 1(50) | 4(9.1) | |
| R | 9(69.2) | 5(71.4) | NA | 3(75) | 1(50) | NA | 1(50) | 19(43.2) | |
| CFZ | S | NA | NA | 3(30) | NA | NA | 2(33.3) | NA | 5(11.4) |
| I | NA | NA | 0(0) | NA | NA | 0(0) | NA | 0(0) | |
| R | NA | NA | 7(70) | NA | NA | 4(66.7) | NA | 11(25) | |
| GEN | S | 5(38.5) | 3(42.9) | 4(40) | 2(50) | 2(100) | 3(50) | 2(100) | 21(47.7) |
| I | 2(15.4) | 1(14.3) | 1(10) | 1(25) | 0(0) | 0(0) | 0(0) | 5(11.4) | |
| R | 6(46.2) | 3(42.9) | 5(50) | 1(25) | 0(0) | 3(50) | 0(0) | 18(40.9) | |
| MER | S | 7(53.8) | 4(57.1) | 6(60) | 3(75) | 2(100) | 4(66.7) | 2(100) | 28(63.6) |
| I | 1(7.7) | 1(14.3) | 1(10) | 0(0) | 0(0) | 0(0) | 0(0) | 3(6.8) | |
| R | 5(38.5) | 2(28.6) | 3(30) | 1(25) | 0(0) | 2(33.3) | 0(0) | 13(29.5) | |
| TET | S | 4(30.8) | 2(28.6) | NA | 1(25) | 1(50) | 2(33.3) | 1(50) | 11(25) |
| I | 2(15.4) | 1(14.3) | NA | 1(25) | 0(0) | 0(0) | 0(0) | 4(9.1) | |
| R | 7(53.8) | 4(57.1) | NA | 2(50) | 1(50) | 4(66.7) | 1(50) | 19(43.2) | |
| DOX | S | 5(38.5) | 3(42.9) | NA | 2(50) | 1(50) | 3(50) | 1(50) | 15(34.1) |
| I | 1(7.7) | 1(14.3) | NA | 0(0) | 0(0) | 0(0) | 0(0) | 2(4.5) | |
| R | 7(53.8) | 3(42.9) | NA | 2(50) | 1(50) | 3(50) | 1(50) | 17(38.6) | |
| CPR | S | 2(15.4) | 2(28.6) | 3(30) | 1(25) | 0(0) | 2(33.3) | 1(50) | 11(25) |
| I | 2(15.4) | 1(14.3) | 0(0) | 0(0) | 1(50) | 0(0) | 0(0) | 4(9.1) | |
| R | 9(69.2) | 4(57.1) | 7(70) | 3(75) | 1(50) | 4(66.7) | 1(50) | 29(65.9) | |
| COT | S | 4(30.8) | 3(42.9) | NA | 2(50) | 1(50) | 2(33.3) | 1(50) | 13(29.5) |
| I | 1(7.7) | 0(0) | NA | 1(25) | 0(0) | 1(16.7) | 0(0) | 3(6.8) | |
| R | 8(61.5) | 4(57.1) | NA | 1(25) | 1(50) | 3(50) | 1(50) | 18(40.9) | |
| CHL | S | 7(53.8) | 4(57.1) | NA | 3(75) | 2(100) | NA | 2(100) | 18(40.9) |
| I | 1(7.7) | 1(14.3) | NA | 0(0) | 0(0) | NA | 0(0) | 2(4.5) | |
| R | 5(38.5) | 2(28.6) | NA | 1(25) | 0(0) | NA | 0(0) | 8(18.2) |
Abbreviations: AM, Ampicillin; PIP, Piperacillin; AUG, Augmentin; CFP, Cefepime; CXT, Cefoxitin; CTR, Ceftriaxone; CRX, Cefuroxime; CFZ, Ceftazidime; GEN, Gentamicin; MER, Meropenem; TET, Tetracycline; DOX, Doxycycline; CPR, Ciprofloxacin; COT, Cotrimoxazole; CHL, Chloramphenicol; NA, not applicable as per CLSI 2020 guideline; S, sensitive; I, intermediate; R, resistant; spp, species.
Multidrug-Resistant Patterns of Bacterial Isolates
Overall, 67.1% (51/76) of bacterial isolates were multidrug-resistant (MDR). MDR isolates contain 27.6% (21/76) Gram-positive bacteria and 39.5% (30/76) Gram-negative bacteria, respectively. More than 68.4% of S. aureus, K. pneumoniae, P. aeruginosa, E. coli, and Citrobacter spp. isolates developed MDR. ESKAPE pathogens (Enterococcus faecium, S. aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp.) showed a higher MDR rate, 70.6% (36/51), than non-ESKAPE isolates (Table 6).
Table 6.
Multi-Drug Resistance Pattern of Bacterial Isolates from Infection Suspected Orthopedic Surgical Wounds at Arba Minch General Hospital
| Bacterial Isolates | Antibiogram Pattern (%) | ||||
|---|---|---|---|---|---|
| R1 | R2 | R3 | ≥R4 | Total | |
| S. aureus (n=19) | 4(21.1) | 5(26.3) | 3(15.8) | 1(5.3) | 13(68.4) |
| CoNS (n=10) | 3(30) | 2(20) | 1(10) | 0(0) | 6(60) |
| E. faecium (n=3) | 0(0) | 1(33.3) | 0(0) | 1(33.3) | 2(66.7) |
| K. pneumoniae (n=13) | 3(23.1) | 4(30.8) | 1(7.7) | 1(7.7) | 9(69.2) |
| P. aeruginosa (n=10) | 3(30) | 3(30) | 1(10) | 0(0) | 7(70) |
| E. coli (n=7) | 1(14.3) | 1(14.3) | 2(28.6) | 1(14.3) | 5(71.4) |
| A. baumannii (n=6) | 1(16.7) | 1(16.7) | 2(33.3) | 0(0) | 4(66.7) |
| Citrobacter spp. (n = 4) | 1(25) | 0(0) | 1(25) | 1(25) | 3(75) |
| Entrobacter spp. (n=2) | 0(0) | 1(50) | 0(0) | 0(0) | 1(50) |
| Proteus spp. (n=2) | 1(50) | 0(0) | 0(0) | 0(0) | 1(50) |
| Total MDR isolates (n=76) | 17(22.4) | 18(23.7) | 11(14.5) | 5(6.6) | 51(67.1) |
Notes: R1, R2, R3, and R4: Resistant to at least one antibiotic from; three classes, four classes, five classes and six and more classes of antibiotics, respectively.
Abbreviations: MDR, multidrug resistant; CoNS, Coagulase Negative Staphylococci; spp, species.
Prevalence of Biofilm Forming Bacterial Isolates
In vitro biofilm producers accounted for 57.9% (44/76) of the 76 bacteria identified. Strong biofilm formation was observed in 27.6% (21/76), whereas moderate and weak biofilm formations were found in 19.7% (15/76), and 10.5% (8/76), respectively. In 42.1% (32/76) of the isolates, no biofilm formation was seen. Gram-negative isolates had 52.3% (23/44) of biofilm formation, compared to 47.7% (21/44) of Gram-positive isolates. All MDR isolates of Gram-positive bacterial isolates showed a strong, moderate, or low level of biofilm formation. On the other hand, isolates of Proteus spp. did not show any biofilm development (Table 7).
Table 7.
Biofilm Formation of Bacterial Isolates from Infection Suspected Orthopedic Surgical Wounds at Arba Minch General Hospital
| Bacterial Isolates | Frequency (n=76) | Biofilm Formation | |||
|---|---|---|---|---|---|
| No (%) | SBF (%) | MBF (%) | WBF (%) | NBF (%) | |
| S. aureus | 19 (25) | 7 (36.8) | 3 (15.9) | 3 (15.9) | 6 (31.6) |
| CoNS | 10 (13.2) | 2 (20) | 3 (30) | 1 (10) | 4 (40) |
| E. faecium | 3 (3.9) | 0 (0) | 2 (66.7) | 0 (0) | 1 (33.3) |
| K. pneumoniae | 13 (17.1) | 4 (30.8) | 2 (15.4) | 2 (15.4) | 5 (38.5) |
| P. aeruginosa | 10 (13.2) | 3 (30) | 2 (20) | 1 (10) | 4 (40) |
| E. coli | 7 (9.2) | 2 (28.6) | 1 (14.3) | 0 (0) | 4 (57.1) |
| A. baumannii | 6 (7.9) | 2 (33.3) | 1 (16.7) | 0 (0) | 3 (50) |
| Citrobacter spp. | 4 (5.3) | 0 (0) | 1 (25) | 1 (25) | 2 (50) |
| Entrobacter spp. | 2 (2.6) | 1 (50) | 0 (0) | 0 (0) | 1 (50) |
| Proteus spp. | 2 (2.6) | 0 (0) | 0 (0) | 0 (0) | 2 (100) |
| Total | 76 (100) | 21 (27.6) | 15 (19.7) | 8 (10.5) | 23 (30.3) |
Abbreviations: CoNS, Coagulase Negative Staphylococci; SBF, Strong Biofilm Former; MBF, Moderate Biofilm Former; WBF, Weak Biofilm Former; NBF, Non-Biofilm Former; spp, species.
Discussion
Surgical site bacterial infection is the most commonly surveyed and common kind of hospital-acquired infection, according to the World Health Organization (WHO).1,30 In this study, the overall prevalence of bacterial isolates from postoperative orthopedic wounds with clinical suspicion of wound infections was 29.4% [95% CI (23.7, 35.1)], which was equivalent to the worldwide and Brazilian reports of OSSI rates ranging between 1.4% and 41.9%,1,2,16 and the previous study done in Muhimbili, Tanzania (25.0%).2 However, our finding was greater than the studies conducted in Manipal, India, ranging between 1.9% and 22.7%,9 Belgrade, Serbia (22.7%),29 Al Khobar, Saudi Arabia (2.6%),12 Chhattisgarh, India (12%),22 and Abbottabad-Pakistan (5.30%).7 On the contrary, our findings were lower than those of studies conducted in various parts of India, with prevalence rates ranging from 68 to 90%,8,15,23,30,31 and studies conducted in Addis Ababa, Ethiopia, with prevalence rates ranging from 54.3% to 82.4%.14,23 The disparity could be explained by the incidence of bacterial etiology and infection prevention efforts in different hospital settings, study design, sample size, and population differences. Despite these factors, another possible explanation for the low rate of infection seen in our study is that the effect of antimicrobials used for surgical prophylaxis, the administration of antibiotics prior to obtaining culture samples, and antiseptics used for cleaning the wounds has been suggested to reduce the risk of infection; and the infection can be broad, including strictly anaerobic bacteria and fungi that really fail to grow by the procedures employed, which could be the major explanation for the absence of bacterial growth in samples taken from orthopedic surgical wounds with the existing clinical signs and symptoms of infection.5,14,23
The rate of orthopedic surgical site infection was found to be statistically related to a variety of parameters in the current investigation. External fixation and open fracture fixation patients had a 4.761 times higher chance of developing orthopedic surgery site infections. Similar investigations in RajaRajeswari, Bengaluru,5 Tehran, Iran,13 Cambridge University,20 and Addis Ababa, Ethiopia10 have documented the rate of infection associated with external fixation. This might be because external fixation increases the risk of infection because the skin barrier has been disrupted, exposing bone and other internal structures to the outside environment through a skin wound.
The usage of orthopedic implants was found to be statistically significant, with a 2.318-fold increased risk of developing OSSIs. Independent research in Muhimbili, Tanzania,2 Tehran, Iran,13 Minas Gerais, Brazil,16 Liestal, Switzerland,18 and Rangaraya, India22 found comparable results. Clinical studies have shown that the presence of a biomaterial (a suture, internal fixation device, or other device) and damaged tissue (dead bone or wounded soft-tissue) makes the nearby tissues vulnerable to infection both immediately and delayed.2,3,18 This is due to a locally acquired granulocyte defect and hematoma, as well as the presence of dead space around the implanted device, which is an ideal environment for bacterial growth, is difficult to penetrate with antibiotics administered postoperatively, and leads to a reduction in the ability of normal defense mechanisms due to tissue devascularization near the wound. Additionally, reaming the bone causes the tissue in the immediate surroundings to die, further limiting blood flow.13,18 Furthermore, biofilm formation also permits bacteria to cling to and survive on implants, which is a major source of antibiotic resistance among biofilm-producing bacteria.18 Thus, scrubbing, draping, and disinfection of surgical equipment and implants, as well as extra oxygenation, preventing hypothermia, optimizing nutrition, continuing intravenous antibiotics for 72 hours, and post-operative wound care, should all be properly monitored.2,18
OSSIs were more likely in orthopaedic surgeries lasting more than two hours. Similar findings were reported in previous investigations in Muhimbili, Tanzania.2,32 Increased tissue and surgical tool exposure to environmental bacteria, surgical team fatigue resulting in poor adherence to aseptic procedures, and a decrease in the patient’s microorganisms’ systemic defenses could all contribute to a significant risk of infection during long surgeries. Similarly, orthopedic patients admitted to the hospital for more than seven days after surgery were four times more likely than patients admitted for less than seven days to develop an orthopedic surgical site infection, implying that the postoperative wound care approach for this group of patients should be reviewed and modified. This is consistent with studies conducted in Belgrade, Serbia3 and Tehran, Iran,13 which found that long-term hospitalization, leads to the development of nosocomial infections. This could be related to invasive medical and surgical procedures, poor post-surgical wound care, and the cleanliness of the hospital’s surroundings, which can serve as a source of bacterial infection via mechanical vehicles.
The diversity of bacterial isolates (S. aureus, K. pneumoniae, E. coli, P. aeruginosa, K. pneumoniae, CoNS, A. baumannii, Citrobacter spp., Enterobacter spp., E. faecalis, and Proteus spp.) observed in the present study was in concordance with the previous studies conducted in different part of India,5,8,9,15,23,31 Belgrade, Serbia,29 Abbottabad-Pakistan,7 and Addis Ababa, Ethiopia.14,23 In line with the previous studies conducted in Addis Ababa, Ethiopia on surgical site infection,14,23 but in contrast to a study done in Kashmir, India,31 the Gram-negative bacilli were predominantly isolated. The high prevalence rate of Gram-negative bacterial isolates could indicate faecal contamination as a result of inadequate personnel hygiene or post-procedural contamination, as well as the high prevalence of Gram-negative bacteria as normal flora in the hospital setting since ESKAPE pathogens were also isolated.33 ESKAPE pathogens, rather than initial surgery site contamination, have emerged as the primary source of postsurgical orthopedic infections associated with hospital-acquired infections. However, due to the presence of bacterial etiology and infection prevention strategies in the hospital, the predominant isolates may vary from different geographical contexts.
In this study, the predominant isolates were S. aureus, followed by K. pneumoniae, P. aeruginosa, and CoNS, which is similar to previous studies.8,14,15,22,23,31 The possible justification for the predominance of S. aureus infection in this study (25%) could be attributed to an endogenous source, such as when the organism colonizes the skin and allows access to deep regions following surgery since it has special receptors for adherence onto the bone being the most common pathogen in osteomyelitis. Exogenously S. aureus can also be spread by the environment, surgical instruments, or via contaminated health-care workers’ hands.14,34,35
In this study, cotrimoxazole, erythromycin, and penicillin resistance were found in 50%, 53.1%, and 75% of Gram-positive bacterial isolates, respectively. However, chloramphenicol (50%), doxycycline (43.8%), cefoxitin (43.8%), and gentamicin (43.8%) were found to be effective against Gram-positive bacterial isolates, which is in line with the findings of previous studies.5,15,23 The predominant isolate, S. aureus, showed 78.9%, 63.2%, and 57.9% resistant to penicillin, cotrimoxazole, and erythromycin, respectively. Likewise, isolated CoNS were shown to be 60% resistant to penicillin. About 57.9% and 40% of isolated S. aureus and CoNS were MRSA and MR-CoNS, respectively, which is in line with studies done in Kakinada, India, where 66.6% of isolates of S. aureus and 60% of isolates of CoNS were MRSA and MS-CoNS, respectively.23 In contrast, our finding is higher than research done in different parts of India where 12%–30% were MRSA and 2% MR-CoNS,15,30 and in hospitals in Bengaluru where 12.5% of MRSA and 2.3% of MR-CoNS were reported.5 Similarly, a systematic review conducted in Maryland, United States of America35 and Groningen, Netherlands36 found lower rates of MRSA among patients with OSSIs, with rates of MRSA ranging from 0.3–2.5% and 39.9%, respectively.
Isolated E. faecalis showed 100% and 66.7% resistant to penicillin and erythromycin, respectively. In contrast to study done in Andhra Pradesh, India,30 in this study, vancomycin-resistant Enterococcus (VRE) was observed in 40% of the isolated species. The increased rate of drug resistance for commonly prescribed antibiotics can be possibly correlated with the frequent use of these antibiotics, their easy availability, and the practice of self-medication, limited diagnostic facilities, inappropriate antibiotic use, and the issuance of prescriptions without susceptibility data, which have been considered the factors associated with this phenomenon.
Gram-negative bacteria isolate showed higher resistant to cefepime (65.9%) and ciprofloxacin (65.9%). However, augmentin (41%), gentamicin (47.7%) and meropenem (63.6%) were effective against Gram-negative isolates. The predominant Gram-negative isolates, K. pneumonia, showed 69.2% resistance to ampicillin, cefepime, cefoxitin, ceftriaxone, cefuroxime, and ciprofloxacin. Likewise, 70% of isolated P.aeruginosa was resistant to cefepime, ceftazidime, and ciprofloxacin. In spite of this, piperacillin (60%) and meropenem (60%) were effective against P. aeruginosa isolates. On the other hand, isolated A. baumannii showed 66.7% of resistant to cefepime, ceftazidime, tetracycline, and ciprofloxacin, while piperacillin (50%), gentamicin (50%), doxycycline (50%), and meropenem (66.7%) were effective against isolated A. baumannii. The overall finding of this study was comparable to previous studies conducted in different parts of India.8,15,22,23,30 The increased use of conventional antibiotics and their dissemination, as well as isolated strain variations, are attributed to the rise in drug-resistant Gram-negative bacterial infections.
The overall multidrug-resistant level of all bacterial isolates was 67.1% (51/76), where Gram-positive and Gram-negative bacteria accounted for 27.6% (21/76) and 39.5% (30/76) of the MDR level, respectively. This finding was higher than that of a study done in Jammu and Kashmir, India among orthopaedic implant infections where the MDR level was 38.1%.31 On the other hand, our finding was in line with the finding of a study conducted on postoperative wound infections at Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia, where the MDR level was 65.5%.14 In contrast, our finding was lower as compared to what has been reported among wound infections in South West Ethiopia, which was 85.2%.33 There could be many explanations for this difference and increased prevalence, including: different bacterial strains, geographic variation, patients’ awareness towards the use of antimicrobials, the difference in hospital control measures, the difference in antibiotic prescribing policies, the easy availability of drugs without a prescription, leading to self-medication, and indiscriminate or prolonged use of common antibiotics, leading to rapid and extensive spread of antimicrobial resistance.14
In the present study, 57.9% of bacterial isolates were in vitro positive for biofilm formation, with 52.3% and 47.7% for Gram-negative and Gram-positive isolates, respectively, where all MDR Gram-positive bacterial isolates were biofilm producers. This result is lower than a study conducted by tube techniques in Southern Odisha, India, which found that 74.3% of biofilm producers.8 In our study, strong biofilm formation was observed in 27.6% of isolates, whereas moderate and weak biofilm formation were found in 19.7% and 10.5% of isolates, respectively, which is comparable with studies reported from Karnataka State, India,15 where 28.1% of isolates were strong biofilm producers. However, only 4.7% of isolates were moderate biofilm producers, which is lower than our result. The difference in biofilm formation patterns among bacterial isolates may be due to differences in strain types, number of bacterial isolates, nature of the surgery (surgery with or without use of implants) where the bacteria isolates, sample sizes, geographic locations, and methodological variations to assess biofilm formation of isolates.
Limitation
The study was limited by the use of conventional culture methods, which were used to identify the species, lacked follow-up study design, and no assays to determine the antimicrobial MIC were done. Due to economic and laboratory set-up constraints, studies on strictly anaerobic bacteria, as well as fungi, are totally constrained. Prior to sampling, the administration of antimicrobial prophylaxis and antiseptics may have influenced the outcome of our investigation. Furthermore, some wound swabs from internal fixations were collected after the administration of local anesthetic, which could have had an antibacterial effect and resulted in erroneous wound culture results.
Conclusion
The magnitude of OSSIs among suspected orthopedic patients was comparable to that of other similar investigations, and the most frequent isolated bacteria were S. aureus and K. pneumoniae. External fixation, use of implants or prostheses, duration of surgery for more than two hours, and post-operative hospitalization for more than seven days were found to be statistically significant associations with OSSIs. The majority of bacterial isolates had higher rates of resistance to commonly prescribed antimicrobials. The overall MDR among isolates was 67.1%, of which ESKAPE pathogens constituted 70.6%. More than half of the bacterial isolates were biofilm-formers. Thus, by strictly adhering to aseptic surgical protocols and wound treatment, adequate precautions should be taken to limit the risk of infection. Furthermore, empirical treatment of nosocomial infections provokes drug resistance; treatment should be based on the results of culture and sensitivity tests, and continuous surveillance for resistant bacteria should be conducted to prevent MDR bacterial infections from emerging and spreading. Finally, further in-depth cohort studies pertaining to the molecular characterization of all bacterial strains, including fungal agents, are warranted.
Acknowledgments
We acknowledge the involvement of Samison Gemechu and Kalab Atinafu, working at Arba Minch University College of Medicine and Health Sciences as technical assistants, in data collection. We appreciate the contributions of health experts at Arba Minch General Hospital during data collection. We appreciate the technical and financial support provided by the Arba Minch University Research Directorate. Our gratitude also goes out to the study participants for agreeing to take part in the research.
Funding Statement
The project was supported by the Arba Minch University Research Directorate.
Abbreviations
CLSI, Clinical and Laboratory Standards Institute; ESBL, Extended Spectrum Beta-Lactamase; MRSA, Methicillin-Resistant Staphylococcus Aureus; MR-CoNS, Methicillin-Resistant Coagulase Negative Staphylococci; MtP, Microtiter Plate; OD, optical density; ODc, cutoff optical density; OSSIs, orthopedic surgical site infections; VRE, Vancomycin-Resistant Enterococcus.
Data Sharing Statement
The data sets analyzed in this study are available from the principal author upon reasonable request due to ethical and confidentiality issues.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
- 1.World Health Organization. Global guidelines for the prevention of surgical site infection. World Health Organization; 2016:135–136. Available from: https://www.who.int/gpsc/global-guidelines-web.pdf. Accessed May 05, 2022. [Google Scholar]
- 2.Kisibo A, Ndume V, Semiono A, et al. Surgical site infection among patients undergone orthopaedic surgery at Muhimbili Orthopaedic Institute, Dar Es Salaam, Tanzania. East Cent Afr J Surg. 2017;22(1):49–58. doi: 10.4314/ecajs.v22i1.7 [DOI] [Google Scholar]
- 3.Starčević S, Munitlak S, Mijović B, Mikić D, Šuljagić V. Surgical site infection surveillance in orthopedic patients in the Military Medical Academy, Belgrade. Vojnosanit Pregl. 2015;72(6):499–504. doi: 10.2298/VSP140224059S [DOI] [PubMed] [Google Scholar]
- 4.Uçkay I, Hoffmeyer P, Lew D, Pittet D. Prevention of surgical site infections in orthopaedic surgery and bone trauma: state-of-the-art update. J Hosp Infect. 2013;84(1):5–12. doi: 10.1016/j.jhin.2012.12.014 [DOI] [PubMed] [Google Scholar]
- 5.Lakshminarayana S, Chavan S, Prakash R, Sangeetha S. Bacteriological profile of orthopedic patients in a tertiary care hospital, Bengaluru. Int J Sci Res. 2013;4(6):2319–7064. [Google Scholar]
- 6.Najjar YW, Saleh MY. Orthopedic surgical site infection: incidence, predisposing factors, and prevention. Int J Med Sci Clin Invent. 2017;4(2):2651–2661. [Google Scholar]
- 7.Shah MQ, Zardad MS, Khan A, Ahmed S, Awan AS, Mohammad T. Surgical site infection in orthopedic implants and its common bacteria with their sensitivities to antibiotics, in open reduction internal fixation. J Ayub Med Coll Abbottabad. 2017;29(1):50–53. [PubMed] [Google Scholar]
- 8.Sarangi SK, Padhi S. Bacteriological profile of post-operative orthopedic implant infections and their antibiotic sensitivity pattern in a tertiary care hospital of southern Odisha. J Dr NTR Univ Health Sci. 2019;8(2):114. doi: 10.4103/JDRNTRUHS.JDRNTRUHS_48_19 [DOI] [Google Scholar]
- 9.Bhat AK, Parikh NK, Acharya A. Orthopaedic surgical site infections: a prospective cohort study. Can J Infect Control. 2018;33(4):227–229. [Google Scholar]
- 10.Abraham Y, Wamisho BL. Microbial susceptibility of bacteria isolated from open fracture wounds presenting to the err of black-lion hospital, Addis Ababa University, Ethiopia. Afr J Microbiol Res. 2009;3(12):939–951. [Google Scholar]
- 11.Bauer L. Arthroplasty. Surgical Technologist. 2010:66. (Available from: https://www.ast.org/pdf/314.pdf. Accessed May 5, 2022). [Google Scholar]
- 12.Al-Mulhim FA, Baragbah MA, Sadat-Ali M, Alomran AS, Azam MQ. Prevalence of surgical site infection in orthopedic surgery: a 5-year analysis. Int Surg. 2014;99(3):264–268. doi: 10.9738/INTSURG-D-13-00251.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hadadi A, Zehtab M, Babagolzadeh H, Ashraf H. Contributing Risk Factors for Orthopedic Device Related Infections in Sina Hospital, Tehran, Iran. Iran Red Crescent Med J. 2011;13(2):117. [PMC free article] [PubMed] [Google Scholar]
- 14.Asres G, Legese M, Woldearegay G. Prevalence of multidrug resistant Bacteria in postoperative wound infections at Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia. Arch Med. 2017;9(4):12. [Google Scholar]
- 15.Fernandes A, Dias M. The microbiological profiles of infected prosthetic implants with an emphasis on the organisms which form biofilms. J Clin Diagnostic Res. 2013;7(2):219. doi: 10.7860/JCDR/2013/4533.2732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ercole FF, Franco LMC, Macieira TGR, Wenceslau LCC, Hind R, Chianca TCM. Risk of surgical site infection in patients undergoing orthopedic surgery. Rev Lat Am Enfermagem. 2011;19(6):1362–1368. doi: 10.1590/S0104-11692011000600012 [DOI] [PubMed] [Google Scholar]
- 17.Zimmerli W. Clinical presentation and treatment of orthopaedic implant‐associated infection. J Intern Med. 2014;276(2):111–119. doi: 10.1111/joim.12233 [DOI] [PubMed] [Google Scholar]
- 18.McConoughey SJ, Howlin R, Granger JF, et al. Biofilms in periprosthetic orthopedic infections. Future Microbiol. 2014;9(8):987–1007. doi: 10.2217/fmb.14.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okike K, Bhattacharyya T. Trends in the management of open fractures. A critical analysis. J Bone Joint Surg Am. 2006;88(12):2739–2748. doi: 10.2106/JBJS.F.00146 [DOI] [PubMed] [Google Scholar]
- 20.Doshi P, Gopalan H, Sprague S, Pradhan C, Kulkarni S, Bhandari M. Incidence of infection following internal fixation of open and closed tibia fractures in India (INFINITI): a multi-centre observational cohort study. BMC Musculoskelet Disord. 2017;18(1):156. doi: 10.1186/s12891-017-1506-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Das RSA, Srivastava P, Pradhan S, Murthy R. Microbial profile and antibiotic susceptibility pattern of surgical site infections in orthopedic patients at a Tertiary hospital in Bilaspur. Int J Sci Study. 2015;3(3):47. [Google Scholar]
- 22.Chandrika S, Kirani SK. Bacteriological spectrum of post-operative orthopedic implant infections and their antibiogram. J Krishna Inst Med Sci Univ. 2016;5(1):20–26. [Google Scholar]
- 23.Mezemir R, Seid A, Gishu T, Demas T, Gize A. Prevalence and root causes of surgical site infections at an academic trauma and burn center in Ethiopia: a cross-sectional study. Patient Saf Surg. 2020;14(1):3. doi: 10.1186/s13037-019-0229-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wayne PA; CLSI. Performance Standards for Antimicrobial Susceptibility Testing CLSI supplements M100. Clinical and Laboratory Standards Institute; 2020. Available from: https://www.nih.org.pk/wp-content/uploads/2021/02/CLSI-2020.pdf. Accessed May 5, 2022. [Google Scholar]
- 25.Cheesbrough M District laboratory practice in tropical countries, part 2: Cambridge university press; 2006. Available from: https://www.medbox.org/preview/5255d6e1-05d4-41a9-beb2-02b60e695ecc/doc.pdf. Accessed May 5, 2022. [Google Scholar]
- 26.Bergey’s. Manual of systematic bacteriology ; 2010. Available from: https://link.springer.com/chapter/10.1007/978-0-387-21609-6_13. Accessed May 5, 2022.
- 27.Hudzicki J. Kirby-Bauer disk diffusion susceptibility test protocol. Am Soc Microbiol. 2009;8(15):55–63. [Google Scholar]
- 28.Stepanović S, Vuković D, Hola V, et al. Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. Apmis. 2007;115(8):891–899. doi: 10.1111/j.1600-0463.2007.apm_630.x [DOI] [PubMed] [Google Scholar]
- 29.Maksimović J, Marković-Denić L, Bumbaširević M, Marinković J, Vlajinac H. Surgical site infections in orthopedic patients: prospective cohort study. Croat Med J. 2008;49(1):58–64. doi: 10.3325/cmj.2008.1.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shabnum M. Microbial profile and antibiotic susceptibility pattern of orthopedic infections in a tertiary care hospital: a study from South India. Int J Med Sci Public Health. 2017;6(5):838–842. [Google Scholar]
- 31.Benazir S, Kakru DK, Khurshid S, et al. Identification, antibiotic susceptibility patterns and biofilm detection of isolates in orthopaedic implant infections. J adv med. 2018;25(5):1–12. doi: 10.9734/JAMMR/2018/38988 [DOI] [Google Scholar]
- 32.Cheng H, Clymer JW, Chen BP, et al. Prolonged operative duration is associated with complications: a systematic review and meta-analysis. J Surg Res. 2018;1(229):134–144. doi: 10.1016/j.jss.2018.03.022 [DOI] [PubMed] [Google Scholar]
- 33.Mama M, Abdissa A, Sewunet T. Antimicrobial susceptibility pattern of bacterial isolates from wound infection and their sensitivity to alternative topical agents at Jimma University Specialized Hospital, South-West Ethiopia. Ann Clin Microbiol Antimicrob. 2014;13(1):1–10. doi: 10.1186/1476-0711-13-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mengesha RE, Kasa BG, Saravanan M, Berhe DF, Wasihun AG. Aerobic bacteria in post-surgical wound infections and pattern of their antimicrobial susceptibility in Ayder Teaching and Referral Hospital, Mekelle, Ethiopia. BMC Res Notes. 2014;7(1):1–6. doi: 10.1186/1756-0500-7-575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korol E, Johnston K, Waser N, et al. A systematic review of risk factors associated with surgical site infections among surgical patients. PLoS One. 2013;8(12):e83743. doi: 10.1371/journal.pone.0083743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Purba AK, Setiawan D, Bathoorn E, Postma MJ, Dik JW, Friedrich AW. Prevention of surgical site infections: a systematic review of cost analyses in the use of prophylactic antibiotics. Front Pharmacol. 2018;18(9):776. doi: 10.3389/fphar.2018.00776 [DOI] [PMC free article] [PubMed] [Google Scholar]