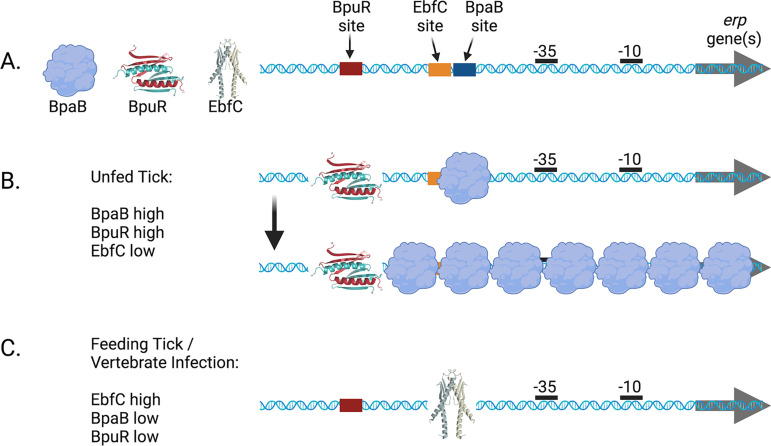
FIG 2.
Mechanism by which B. burgdorferi controls transcription of erp operons. (A) All erp operons include a 5′ operator that consists of a BpaB-binding site, two to three EbfC-binding sites, and a BpuR-binding site. The BpaB- and EbfC-binding sites are adjacent, and the two proteins compete for binding to the DNA. (B) In the midgut of an unfed tick, or under slow-replication culture conditions, BpaB and BpuR levels are elevated, while EbfC levels are low. BpaB binds to its high-affinity binding site and then spreads along the DNA, stabilized by protein-protein interactions. The presence of BpuR enhances transcription repression by BpaB, possibly by influencing the direction of BpaB multimerization. (C) In a feeding tick, during vertebrate infection, or during culture under rapid replication conditions, EbfC levels are high, while BpaB and BpuR levels are low. Binding of EbfC to erp operator DNA blocks binding by BpaB, freeing the promoter for recognition by RNA polymerase. Promoter −35 and −10 elements are indicated by solid black bars 5′ of the open reading frames (ORFs). The illustrated structures of BpuR and EbfC are adapted from the solved and modeled proteins (123, 128, 131).