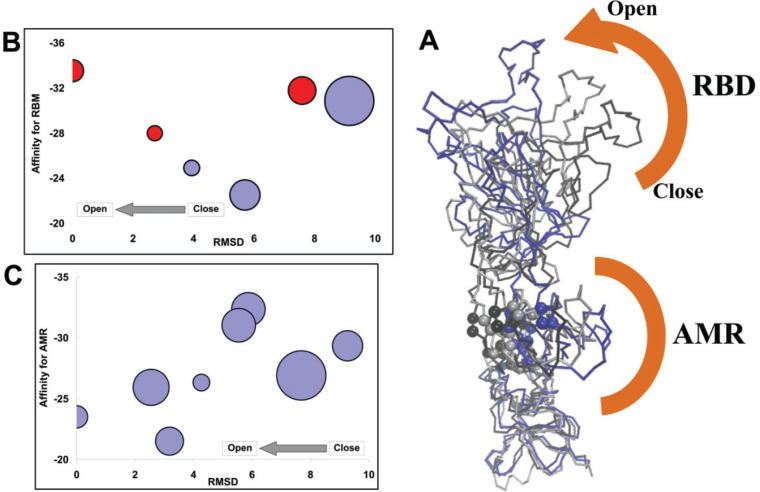
Figure 13.
Results of hepcidin25 interaction with spike in different states. (A) Output of the TMD simulation for sampling the spike structure from closed to open states. The most important residues of the AMR, P > 0.5, are declared by sphere. (B) SWARM-predicted affinity of hepcidin25 for the RBM of spike chains along the transition from closed to open states. The horizontal axis represents the distance of the state to the open conformation of spike by computing the RMSD between the considered frame and the target structure in TMD. (C) Same story but for affinity between hepcidin25 and the AMR in monomeric spike. The size of the circle (B, C) correlates with the size of the SWARM suggesting the top cluster corresponds to the considered representative introduced receptor.