Abstract
Hereditary transthyretin amyloidosis (ATTRv) is a multisystemic, rare, inherited, progressive and adult-onset disease, affecting the sensorimotor nerves, heart, autonomic function and other organs. The actual scenario of pharmaceutical approaches for ATTRv amyloidosis includes five main groups: TTR stabilizers, TTR mRNA silencers, TTR fibril disruptors, inhibitor of TTR fibril seeding and gene therapy. Patisiran is a small, double-stranded interfering RNA encapsulated in a lipid nanoparticle, able to penetrate into hepatocytes, where it selectively targets TTR mRNA, reducing TTR production. We report and discuss 9 cases of different patients with ATTRv amyloidosis successfully managed with patisiran in the real clinical practice. Literature data, as well as the above presented case reports, show that this drug is effective and safe in improving both neurological and cardiovascular symptoms of ATTRv amyloidosis, and to maintain a good QoL, independently form the stage of the disease and the involved mutation. Recent studies correlated improved functional and biochemical outcomes with a regression of amyloid burden, especially at the cardiac level. Today, patisiran can be considered a valid therapeutic option for the management of patients with ATTRv amyloidosis and polyneuropathy and cardiovascular symptoms.
Keywords: hereditary transthyretin amyloidosis, patisiran, real-life, case reports
Introduction
Hereditary transthyretin amyloidosis (ATTRv), also known as familial amyloid polyneuropathy (FAP), is a multisystemic, rare, inherited, progressive and adult-onset disease, affecting the sensorimotor nerves, heart, autonomic function and other organs (gastrointestinal tract, eyes, kidney, connective tissues).1–3 This autosomal-dominant disorder is associated with over 100 different missense mutations that make the transthyretin (TTR) tetramer unstable: as a consequence, a misfolded variant of TTR protein aggregates, generating amyloid fibrils, which accumulate as protein deposits in multiple body organs and tissues.1–3
ATTRv amyloidosis is a heterogeneous disease associated with a wide range of clinical manifestations that may present in varying degrees and combinations, mainly neuropathic or cardiac phenotypes. The main clinical features of ATTRv amyloidosis are carpal tunnel syndrome, peripheral sensory-motor neuropathy, cardiovascular manifestations (conduction blocks, cardiomyopathy, arrhythmia), autonomic manifestations (orthostatic hypotension, recurrent urinary tract infections, sexual dysfunction, sweating abnormalities) and gastrointestinal manifestations (nausea, vomiting, diarrhea, severe constipation, alternating episodes of diarrhea and constipation, unintentional weight loss); central nervous system manifestations (such as headache, progressive dementia, ataxia, seizures), ocular involvement (vitreous opacification, glaucoma) and nephropathy (proteinuria, renal failure) may rarely occur.3 Potential “red-flag” symptom clusters that may warn of a diagnosis of transthyretin familial amyloid polyneuropathy are a progressive symmetric sensory-motor neuropathy plus ≥1 of the following: family history, early autonomic dysfunction, unexplained weight loss, gastrointestinal complaints, cardiac hypertrophy, arrhythmias, ventricular blocks, cardiomyopathy, bilateral carpal tunnel syndrome, renal abnormalities and vitreous opacities.3
The progressive decline of neurological functions, which is variably associated with disabling cardiological, sensori-motor, and autonomic symptoms, negatively affects the patient’s quality of life and requires increasing involvement of relatives in the patient’s daily life.4 Hence, specific scales are commonly used to define the severity of polyneuropathy in ATTRv amyloidosis: the familial amyloid polyneuropathy stage (FAP), and polyneuropathy disability (PND) scores with FAP stage 1 and PND score 1 depicting a patient with mild neuropathy and preserved ambulation, while FAP stage 3 and PND score 4 describe bedridden patients unable to stand up.4
The therapeutical management of ATTRv amyloidosis is based on symptomatic therapy, anti- amyloid therapy to prevent further production of amyloid deposits and treatment of cardiac, renal and ocular involvement, including heart or kidney transplantation.2 Hence, a multidisciplinary approach is on demand.5 In the last years, several disease-modifying therapies have been developed: the actual scenario of current pharmaceutical approaches for ATTRv amyloidosis includes five main groups: TTR stabilizers (tafamidis, diflunisal, Epigallocatechin-3-Gallate, tolcapone, benzbromarone), TTR mRNA silencers (inotersen, patisiran, vutrisiran), TTR fibril disruptors (doxycycline, tauroursodeoxycholic acid, monoclonal antibodies), inhibitor of TTR fibril seeding and gene therapy.2,6
Patisiran is a small, double-stranded interfering RNA encapsulated in a lipid nanoparticle: when the complex enters into hepatocytes, it selectively targets TTR mRNA, reducing both ATTRv and ATTRwt production.7 The phase III placebo-controlled APOLLO study evaluated efficacy and safety of patisiran (0.3 mg per kilogram of body weight) versus placebo once every 3 weeks at 18 months in 225 patients with different severity of disease. All end points were better with patisiran than with placebo respect to baseline, regardless of the stage of the disease, the associated TTR variant and the age of onset: modified Neuropathy Impairment Score+7 (mNIS +7, primary endpoint: 56% vs 4%), Norfolk QoL scores (51.4% vs 10.4%), gait speed (53% vs 13%) and Composite Autonomic Symptom Scale-31 (COMPASS-31) measure of autonomic symptoms. Besides, the use of patisiran was associated to an 81% reduction in serum TTR level. Finally, patisiran significantly improved quality of life, nutritional status and activities of daily living.8 An analysis of several cardiac parameters in a pre-specified cardiac subpopulation of APOLLO study showed a beneficial effect on cardiomyopathy, suggesting that patisiran could halt or reverse the progression of cardiac symptoms of ATTRv amyloidosis patients.9 A recently published interim 12-month analysis of the global OLE study, including patients from APOLLO study and phase 2 OLE study, showed that patisiran is able to maintain a long-term efficacy with an acceptable safety profile in patients with ATTRv amyloidosis and polyneuropathy. These data also support the importance of an early treatment to halt or reverse the progression of polyneuropathy, dysautonomia, disability, malnutrition and QoL impairment.10
In this publication, 9 patients with ATTRv amyloidosis treated with patisiran in Italian reference centers will be discussed. All patients gave own consent to the publication of their clinical data in anonymous form for scientific and educational purposes, but institutional approval was not required to publish the case details.
Case Reports
Two Cases of ATTRv Amyloidosis with a Different Onset: FAP 2 and FAP 1
FAP 2 ATTRv Amyloidosis
A 69-year-old man with an history of arterial hypertension and cataract presented in October 2019 for an insidious onset of distal weakness over a year, beginning in the lower limbs and progressively involving the upper limbs. About 6 months after the onset, he started to complain pain and paresthesia to hands and feet, other than gastrointestinal upset (nausea, vomiting, diarrhea alternating to constipation), erectile dysfunction and genital hypoesthesia. His father died for complications related to kidney failure. The first neurologic examination showed a symmetric distal upper and lower limb weakness, absent ankle reflexes and distal sensory loss. He was unable to walk on his heels or toes. Motor conduction studies showed a reduced compound muscle action potential (CMAP) in the lower limbs, especially on the tibial nerve (0.846 mV on the right, 0.264 mV on the left), with a slight reduction in conduction velocity and a mild increase in latency of F-responses; in upper limbs, a slowed conduction velocity of the median nerve was observed. Sensory studies showed a reduced amplitude of sensory nerve action potentials (SNAPs) for median and ulnar nerves bilaterally, with a slight increase in distal latencies and conduction velocity. Lumbar puncture showed 1.6 cells/mm3 and a protein level of 476 mg/L. In the suspect of a symmetric distal variant of chronic inflammatory demyelinating polyneuropathy (CIDP), the patient received intravenous (iv) immunoglobulin and prednisone, without any benefit. Hence, a genetic test for ATTRv found a Phe64Leu (p.Phe84Leu) mutation of the TTR gene. Additional investigations were carried out to assess the extent and severity of organ involvement and to monitor disability. The patient retained walking ability but he needed assistance [familial amyloid polyneuropathy (FAP) stage 2] and had a polyneuropathy disability (PND) score III. The NIS value was 55 and its strength sub-score, NIS-W, was 19. Autonomic symptoms and quality of life have also been assessed through the COMPASS-31 questionnaire and the Norfolk Quality of Life-Diabetic Neuropathy (QoL-DN) questionnaire, scoring 36 and 93, respectively. Furthermore, a Karnofsky performance status of 60%, a body weight of 83 kg and 6-min walking test (6MWT) result of 130 mt were recorded. Renal and hepatic function were normal, while urinalysis showed urinary tract infection; the values of the cardiac serum biomarkers, N-terminal prohormone of brain natriuretic peptide (NT-proBNP) and T troponin, were high (920 pg/mL and 103 pg/mL, respectively). Bone scintigraphy with 99mTchydroxydiphosphonate (Tc99-HMDP) did not show any cardiac uptake. An electrocardiogram showed sinus rhythm, first degree atrioventricular block, right branch block with left anterior hemiblock. An echocardiogram showed an increased wall thickness (SIV 12 mm), a normal left ventricular function (LVEF 55%), a I degree diastolic dysfunction, a slight left atrial dilation, a normal right ventricular function, a dilation of the ascending aorta, a slight reduction in systolic tissue velocities (septal and lateral s’ 0.06 m/ sec and 0.08 m/ sec respectively). Speckle tracking echocardiography showed a slight reduction of myocardial deformation indices (global longitudinal strain - GLS - 18%) with apical sparing. Unfortunately, cardiac MRI was not performed due to claustrophobia. Patisiran was started on June 2020 and continued until now without any adverse event. Two months later, the patient complained a greater difficulty in walking, attributed to a stenosis of the lumbar canal (documented by spinal imaging). On March 2021, despite the ambulation was possible with assistance (FAP stage: 2), the distal weakness and sensory loss were stable (NIS: 49, NIS-W: 19). Also, an improvement of body weight (86.5 kg), symptoms (COMPASS-31: 19, Norfolk QoL-DN: 72) and more than 40% of 6MWT (160 mt vs 130 mt) was observed. Urinalysis showed microalbuminuria; the cardiologic revaluation showed frequent premature ventricular beats, without any significant change in the echocardiogram; the indices of clinical and subclinical systolic function of the left ventricle remained unchanged. On June 2021, the patient recovered from the spinal lumbar stenosis with benefit on ambulation and further improvement on 6MWT (185 mt), while neurophysiological findings were slightly worsened on the lower limbs (not elicitable CMAP of tibial nerves). In the meantime, our patient received Spikevax mRNA-1273 vaccine (Moderna) on 7th of August 2021, without any adverse event and stable ATTRv symptoms. To date, the patient is COVID-19 free.
FAP 1 ATTRv Amyloidosis
On January 2021, a 62-year-old man with hypertension, hypercholesterolemia and tabagism accessed to out Department for an insidious onset of distal sensory loss over in the lower limbs since a couple of years, recently spreaded to the upper limbs. He was very anxious about his condition because five brothers had a severe polyneuropathy (one was in a wheelchair) and his father had died for a cardiac disease. One of his brothers recently received a diagnosis of hereditary ATTRv amyloidosis. Hence, a genetic testing showed a Phe64Leu (p.Phe84Leu) mutation in the TTR gene. The first neurologic examination showed symmetric distal lower limb weakness, reduced ankle reflexes and distal sensory loss; he was able to walk (FAP stage: 1). Nerve conduction studies showed a bilateral carpal tunnel syndrome and a sensory axonal polyneuropathy with symmetric reduction of SNAP amplitude of sural nerve. The patient did not have any other symptoms of the disease. Tc99-HMDP bone scintigraphy did not reveal any cardiac uptake; he had no cardiac involvement and a normal cardiac function, with normal values of NT-proBNP (41 pg/mL) and T troponin (3 pg/mL). Electrocardiogram showed sinus rhythm without anomalies of atrioventricular conduction and echocardiography a normal biventricular function (LVEF 64%), a normal wall thickness, normal tissue velocities, normal indices of myocardial deformation (GLS - 21%). The NIS score was 19 and NIS-W was 5. The COMPASS-31 and Norfolk QoL-DN questionnaires scored 22 and 18, respectively. Furthermore, a Karnofsky performance status of 70%, body weight of 78 kg and 6MWT of 286 mt were recorded. A full assessment of common causes of polyneuropathy was performed, without any significant result. Hence, patisiran was started on March 2021 and continued until now without any adverse event. The patient received two doses of COVID-19 mRNA BNT162b2 (Pfizer-BioNTech) vaccine on 11th of May and 16th of June, without any adverse event and with stable symptoms of ATTRv. On September 2021, he had unimpaired ambulation (FAP stage 1), unchanged body weight and improved distal sensory loss and muscle strength (NIS: 4, NIS-W: 1). Besides, an improvement of almost 40% in 6MWT (395 mt vs 286mt) and in other symptoms and quality of life (COMPASS-31: 6, Norfolk QoL-DN: 6) with an increased Karnofsky performance status of 80%., was observed. The cardiological tests showed no significant changes in electrocardiographic and echocardiographic parameters, functional capacity unchanged, minimal improvement in subclinical indices of systolic function of the left ventricle. To date, the patient is COVID-19 free.
Discussion
We reported data on 2 patients with genetically confirmed ATTRv amyloidosis followed at the referral center for amyloidosis in the Western Sicily, the University Hospital of Palermo. In the last years, the increased attention to this disease has led to a significant increase in diagnoses compared to the recent past, with an increased prevalence compared to 2015.11 We believe that the increased attention in Sicily was related to the availability of new specific drugs, able to change the prognosis and the life expectancy of ATTRv patients.1 Our patients were treated with patisiran in the post-marketing phase (after February 2020) in Italy; unfortunately, the first months after commercialization of patisiran overlapped with the first wave of SARS2-COVID pandemic with relevant difficulties and some delay in the access to therapies.12,13 In the first case, a patient misdiagnosed with CIDP came our attention with a FAP stage 2 severe polyneuropathy, after an unsuccessful IVIg trial. Electrocardiographic and echocardiographic findings suggested the hypothesis of a cardiac amyloidosis. However, these signs, with the exception of apical sparing, are not specific for cardiac amyloidosis, also considering the hypertension. Thus, the echocardiogram findings alone cannot confirm the presence of cardiac amyloidosis. After the introduction of patisiran, motor scores, quality of life and ambulation speed significantly improved, with a benefic effect and good satisfaction reported by the patient. However, no significant effect on cardiac function was observed after a follow-up of 16 months. The second patient is the brother of another patient followed in our institution, who came early to genetic testing, thus taking advantage of early treatment with patisiran for the FAP stage 1 disease. In this case, despite a less severe impairment of neurologic functions, a more significant improvement has been recorded compared to the first case. Indeed, all scores for polyneuropathy reduced with a very good outcome on quality of life and patient’s independence. The second patient did not have a cardiomyopathy. Finally, in our limited experience, vaccines against COVID-19 resulted safe in ATTRv patients treated with patisiran.
ATTRv Amyloidosis with Polyneuropathy and Glu89Gln Mutation
The 55-years-old male patient referred a positive familial history for ATTRv: a sister and a father’s cousin received a diagnosis of ATTRv (mutation Glu89Gln). He was symptoms free until 2011, when numbness at the first three fingers of both hands appeared: a diagnosis of bilateral carpal tunnel syndrome was done. This symptom remained isolated until 2018, when he developed diarrhea associated with unexplained weight loss (10 kg). Genetic testing, performed in July 2019, confirmed the presence of Glu89Gln (p.Glu109Gln) mutation on the TTR gene. At that time, clinical and neurophysiological evaluations were not consisting with a diagnosis of polyneuropathy, and no specific treatment for ATTRv was started. Rifaximin 200 mg x 3/die and probiotics were prescribed for the treatment of the diarrhoea. On January 2020, the patient referred asthenia, moderate effort’s dyspnea, sporadic hands’ paresthesias and lower limbs’ cramps, diarrhoea and an additional weight loss (-17 kg in total, since 2017). Neurological examination showed hyporeflexia and distal hypoesthesia at lower limbs. The NIS value was 8, PND score was I and FAP stage was I. Electromyography with nerve conduction velocities showed a bilateral carpal tunnel syndrome and a reduction of cMAP of right peroneal and tibial nerves. Electrocardiogram showed signs of anterior and inferior pseudonecrosis. Echocardiography showed a hypertrophic cardiomyopathy (SIV: 15.7 mm; left ventricular posterior wall (LVPW): 14.6 mm) with I degree diastolic dysfunction; the ejection fraction (EF) was 52%. NT-proBNP value was only slightly above the normal range. Due to the fact that fat biopsy was positive for amyloidosis, a therapy with tafamidis meglumine was started. On December 2020, the patient’s weight was 62 kg (−26 kg since the beginning of the disease), associated to chronic diarrhea (7–8 events/day), frequent vomiting episodes, gastroesophageal reflux, loss of appetite, severe asthenia at 4 limbs and severe orthostatic hypotension. At neurological evaluation, patient was able to walk for few meters with bilateral support. The patient had depressed mood, dysphonia, diffuse muscular hypotrophy, moderate/severe proximal and distal hyposthenia at 4 limbs, areflexia, and distal hypoesthesia at upper and lower limbs. NIS was 61, PND score 3b and FAP stage II. He was unable to complete the 6MWT test for the severe dizziness. The logopedic examination showed moderate dysphonia and ipophonia with reduced phonatory times. The psychological evaluation concluded for post-stress depression. Fibrolaryngoscopy showed a vocal cords paresis and signs of gastroesophageal reflux. Echocardiography showed an EF value of 45%, a reduced systolic function, a I degree diastolic dysfunction and a mild pericardiac effusion. The NT-proBNP value was 1682 pg/mL. Due to the clear progression of the disease, the therapy with tafamidis was stopped and a treatment with patisiran was started, together with paroxetine 20 mg/die, omeprazole 40 mg/die, levosulpiride 25 mg/die and loperamide 2 mg x 3/die. Six months later, (June 2021) he gained 5 kg with less diarrhea episodes (4 attacks/die) and orthostatic hypotension symptoms; post-prandial nausea and vomiting were absent. He was able to stand for considerably longer period of time and completed the 6MWT walking 215 meters with bilateral support. At the last visit (November 2021), the weight further increased to 73 kg and the patient reported occasional episodes of diarrhea (1–2 times a day); no symptoms attributable to orthostatic hypotension were reported. Supine blood pressure was 120/80 mm/Hg, and after 3 minutes of standing it reduced only of 10 mm/hg (110/80 mm/Hg). Neurological examination showed almost normal proximal muscles strength (MRC 5-) and mild distal weakness of toe extensors (MRC 4). The gait was slightly ataxic, possible on the heels with some difficulty. He was areflexic. Distal hypoesthesia was present at lower limbs; the NIS value was 25, the PND score 2 and the FAP stage I. He completed the 6MWT walking 282 meters, without any support.
Discussion
Our patients had a Glu89Gln mutation, one of the most aggressive variants with the onset around the age of fifty.14 He developed the first symptoms of disease in 2018, but the regular follow-up showed the onset of the first signs of polyneuropathy only about 18 months later, allowing a treatment with a tetramerous stabilizer (tafamidis). Unfortunately, after almost a year, his clinical conditions worsened: tafamidis was stopped and patisiran was started. In spite of his severe clinical condition at that time (he was able to walk only with bilateral support, presented proximal and distal weakness at 4 limbs and severe symptoms of autonomic nervous system involvement), the clinical response to the new drug was brilliant (Table 1). Diarrhoea and orthostatic hypotension reduced and then almost disappeared, motor strength improved greatly and he was again able to walk independently (PND score 2), with increased physical resistance. As showed by other clinical evidences,15 patisiran has a promising efficacy even in late stage of ATTRv disease and its use should be advised in patients with severe clinical conditions.
Table 1.
Outcome Measures Changes Between Baseline and Last Follow-Up
| Baseline | Last Follow-Up | |
|---|---|---|
| Weight | 62 | 73 |
| PND score | 3b | 2 |
| FAP stage | 2 | 1 |
| NIS | 61 | 25 |
| 6MWT | 0 | 282 |
| Diarrhoea | +++ | + |
| Orthostatic hypotension | +++ | / |
Notes: +: mild; ++: moderate; +++: severe; /: absent.
Abbreviations: 6MWT, 6-min walking test; FAP, familial amyloid polyneuropathy; NIS, Neuropathy Impairment Score; PND, polyneuropathy disability.
ATTRv Amyloidosis with Ile68Leu Mutation and a Combined Orthotopic Heart and Liver Transplantation
This is the case of a male worker with a previous history of right carpal tunnel syndrome and “in situ” rectal cancer surgically treated at the age of 39 and 46 respectively. Patient had no complications and underwent regular follow-ups. At the age of 47 years, during a routinary occupational health control, the physical examination found “cardiac abnormalities”: the patient was sent to a cardiological consultation. The electrocardiography (ECG) documented decreased QRS voltage and echocardiogram displayed an unknown moderate hypertrophic cardiomyopathy with ejection fraction < 35%. Due to these findings and the positive family history (father died at the age of 61 for heart attack), an automatic defibrillator was implanted. Other tests included a 99mTc-DPD Tc99-HMDP bone scintigraphy, which showed a pathological accumulation at heart level (Perugini score 2),16 and gene testing for TTR gene mutations, which documented a Ile68Leu mutation. Due to rapid and progressive worsening of cardiological parameters, patient underwent CHLT on Feb 2009 (age 50). Patient’s condition improved notably after this procedure and he remained stable for many years. At the age of 56 years (2015), the patient had pain at rest in lower limbs and a neurogenic claudication. Neurological tests showed a slight bilateral ptosis and absent deep tendon reflexes without sensory or motor deficits; gait and balance were unremarkable. Electrophysiological studies documented a bilateral carpal tunnel syndrome and a sensory-motor axonal neuropathy and the lack of sympathetic skin reflexes (SSR) during breathing and reduced SSRs due to electric shock on four limbs. The autonomic nervous system assessment demonstrated denervated heart with no orthostatic hypotension. Since 2017, patient reported a progressive weight loss (from 74 to 60 in the following 4 years) without diarrhea. Gastroscopy and colonoscopy were normal. A neurological re-evaluation at the age of 58 years confirmed the slight bilateral ptosis and the absence of deep tendon reflexes, with 25% weakness of quadriceps and psoas in right lower limb and a broad base ataxic walk. Lumbosacral spinal magnetic resonance (MRI) showed a vertebral canal stenosis, treated with nonsurgical treatments with partial relief. On subsequent neurological examinations (2018), the sensory deficits worsened and patient developed vibration deficits in the four limbs, as well as hands and feet numbness and light touch hypoesthesia. Patient gait become wider based and ataxic with repeated falls. Electrophysiological studies showed the worsening of the polyneuropathy with mixed (axonal and demyelinating features) in lower limbs. Since 2020, despite regular cardiological follow-ups, the neurological status of the patient further worsened, with persistent paresthesia and pain in hands and feet, difficulties in finger movements (he become unable to open a water bottle, or tiding his shoe laces) and postural instability. He also complained dizziness/vertigo on standing up and his walking capacity was severely reduced, being able to walk for less than 500 mt without stops. He also reported progressive sweating loss and severely reduced gastrointestinal transit with constipation alternated with weakly bouts of diarrhea. At this stage, gastric biopsies showed a Congo red staining and a gastroduodenal amyloidosis was diagnosed. Further electrophysiological studies performed on April 2021 showed a severe mixed polyneuropathy and absent SSRs on four limbs. Autonomic function tests documented severe orthostatic hypotension, absent blood pressure responses to the Valsalva maneuver and isometric exercise. The global NIS score was 64 (NIS weakness total score 32), PND II and the Karnofsky performance status was 80. Due to this rapid deterioration of the neurological status of the patient, patisiran infusions every 3 weeks were started; the treatment was well tolerated and no side effects were reported. After 9 months of treatment, patient reported stable neurologic functions and an improvement of the gastrointestinal function with less frequent bouts of diarrhea and no further weight loss. At the last neurological follow-up (September 2021), the global NIS score was 41 (NIS weakness total score 22), PND II and the Karnofsky performance status was 80. Patient was still autonomous and able to reach the hospital alone by train from 100 km away.
Discussion
Patients with ATTRv amyloidosis often experience disease progression after orthotopic liver transplant, partially due to a continuous production and deposition of wild type TTR amyloid and misfolding of wild type TTR (ATTRwt) depositing on existing amyloid foci. Newly approved TTR gene silencing therapies (inotersen and patisiran) significantly suppress the production of both mutated TTR (ATTRv) and TTRwt, and significantly delay the progression of neuropathy in ATTRv patients. Patisiran was beneficial and well tolerated in our ATTRv patient who progressed despite orthotopic heart and liver transplantation.
A Brilliant Therapeutical Response in Patisiran-Treated ATTRv Glu89Gln Cardiomyopathy
A 51-years-old Sicilian woman presented to our attention with shortness of breath elicited by physical exercise, occasional lower limbs pitting oedema, bilateral paresthesia of hands and pain started several months before. Her mother was diagnosed ATTRv in her sixties, so the patient already knew to be a carrier of a Glu89Gln (p.Glu109Gln) mutation, because she was tested for TTR several years before when still asymptomatic. Except a bilateral positive Phalen sign, the neurological examination was normal with a NIS value of 0.17 The nerve conduction study (NCS) showed a bilateral mild carpal tunnel syndrome, without any sign of polyneuropathy, while the Sudoscan® test18 had normal skin chloride electro-conductance (ESC) values at both feet and hands. At the time of first visit, she was classified as NYHA class II: the ECG was normal, while cardiac ultrasonography showed a concentric left ventricular hypertrophy with an interventricular septum thickness (IVST) of 17 mm and 45% of ejection fraction. NT-pro-BNP and TnTI were both raised (respectively 6456 pg/mL and 56 ng/L). Tc99-HMDP bone scintigraphy confirmed a myocardial TTR deposition (Perugini score 2).16 Accordingly, a treatment with the TTR tetramer stabilizer tafamidis 20 mg/day was started, associated to furosemide 25 mg/day.
After 6 months of follow-up, where a slight cardiac symptoms amelioration was observed, the patient returned to our attention complaining again shortness of breath for small efforts, bilateral lower limb oedema and nycturia. Moreover, she reported 6–7 episodes of diarrhea per month as well as an occasional sensation of burning feet. Her cardiac ultrasound parameters were unvaried, NT-pro-BNP was 5789 pg/mL, TnTI was 52 ng/L. The neurological examination was normal (NIS 0). A new NCS showed no signs of polyneuropathy, while Sudoscan showed a mild reduction of ESC at feet (52 microSiemens). An ankle-thigh skin biopsy showed a moderate distal skin denervation with 7.3 fiber/mm at ankle.19 The dose of furosemide was increased to 50/25 mg at alternate days, and a switch to the TTR-suppressor siRNA Patisiran was proposed. One month later, the patient started patisiran 0.3 mg/kg every 3 weeks. Six months after the treatment switch, patient reported a progressive amelioration of cardiac symptoms, started approximately after 4 months of patisiran treatment. The drug was well tolerated, without any adverse effect. Now, she was classified NYHA class I and the NT-pro-BNP value 3103 pg/mL. The frequency of diarrhea episodes reduced (2–3 episodes per month), while burning feet sensation remained almost the same in term of intensity and frequency. The dose of furosemide was reduced to 25 mg/day. At the last available follow-up, one year after Patisiran introduction, the patient remained asymptomatic from a cardiological point of view (NYHA class I), with a NT-pro-BNP value of 3058 pg/mL, TnTI 35 ng/L, and an ejection fraction of 45%. IVST was 17 mm. No further adjustments of the diuretic therapy were necessary. The neurological examination was normal (NIS 0) and diarrhea episodes almost disappeared. Burning feet remained an occasional complaint.
Discussion
The Glu89Gln mutation in TTR leads to a peculiar form of ATTRv particularly present in Italy and Bulgaria, where cardiomyopathy could represent both the main and the initial clinical manifestation of the disease.20 Our case shows that an early treatment with patisiran in a cardiac predominant ATTRv Glu89Gln patient could be effective in terms of symptom control and cardiomyopathy biomarker improvement. In Italy, patisiran is approved for the treatment of ATTRv related polyneuropathy with FAP stage of at least 1.21 Thus, the Sudoscan test and the skin biopsy could provide an objective demonstration of skin denervation and autonomic dysfunction in a patient with only mild small fiber neuropathy symptoms and normal NCS. Our cardiological outcomes are similar to those of the APOLLO trial,9 underlining how the use of patisiran is be safe and effective in the treatment of ATTRv cardiomyopathy. Finally, our patient showed a consistent reduction of gastrointestinal symptoms, in line with sub-analysis of the APOLLO trials concerning the autonomic outcomes.22
A Case of Cardiac ATTRv Amyloidosis: The Relevance of Multidisciplinary Approach
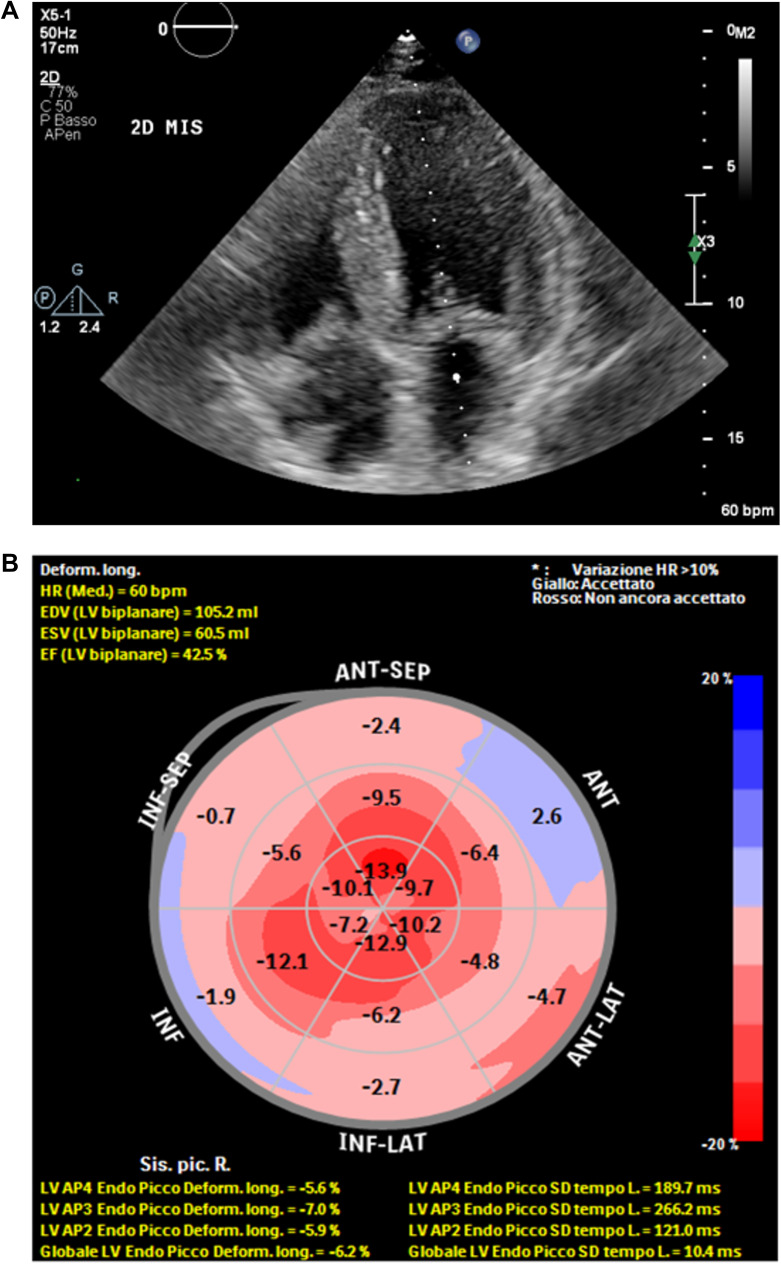
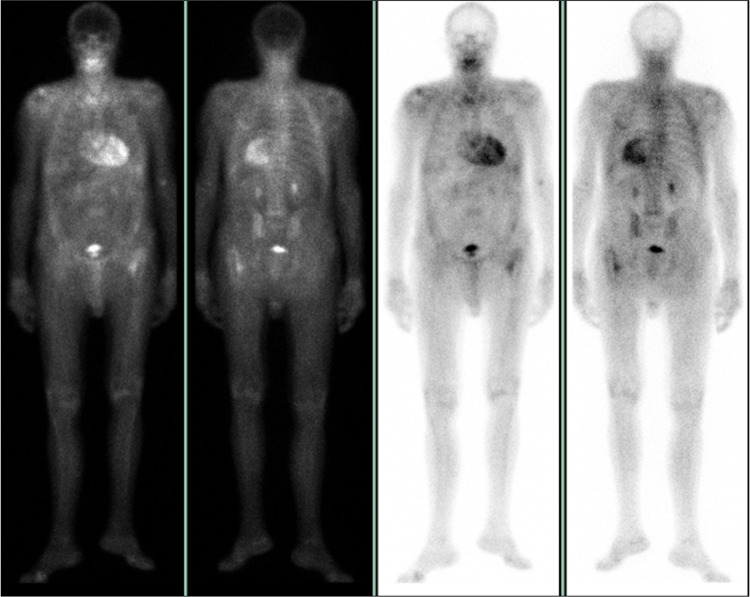
A 75-years-old Caucasic male patient was admitted to the Emergency Department (ED) of our Hospital in July 2017 due to a sudden onset of dyspnea, chest tightness and general asthenia. The medical history was positive for hypertension, benign prostatic hyperplasia, monoclonal gammopathy of undetermined significance (MGUS, IgG and IgM with k light chain) and active smoke; he undergone bilateral carpal tunnel surgery in 2006. He had no siblings and his familial medical history was positive for cardiopathy (his paternal grandfather). The laboratory tests (notably troponin I dosage and electrolytes), performed in ED, were negative and the ECG showed sinus bradycardia with second-degree atrioventricular block. For this reason, he was hospitalized in the Cardiology Unit. An echocardiogram showed a moderately dilated left atrium, a mild mitral and moderate tricuspid insufficiency with pulmonary artery pressure values of 52 mmHg, and a mildly congested inferior vena cava. He was then treated with a dual chamber pace-maker implantation and discharged with an antihypertensive therapy (angiotensin-receptor blocker/calcium channel blocker). Few weeks later, he was re-hospitalized for a perforation of the heart wall caused by the catheter of the device, which was extracted and repositioned; collaterally, he was diagnosed a venous thromboembolism complicated by pulmonary embolism, treated with NOACs. Subsequently, the patient was fine until December 2019, when a worsening of dyspnea occurred (NYHA IIb/III) despite a diuretic therapy. A further echocardiogram showed marked hypertrophy (interventricular septal thickness: 20 mm, posterior wall of left ventricle: 14 mm) with slightly reduced pump function (EF 45%), signs of increased filling pressure and a severe reduction of longitudinal deformation indices (global longitudinal strain, GL2D-strain: −6.2%), with an “apical sparing pattern” specific for cardiac amyloidosis (Figure 1A and B).23 Under a clinical suspect of AL amyloidosis and considering the underlying MGUS, a biopsy of the iliac crest bone marrow was performed, which ruled-out the presence of amyloid, as well as of lymphoma or multiple myeloma. At this point, a Congo red histochemical investigation and polarized light test performed on abdominal fat tissues showed the presence of amyloid; however, no immunofluorescence typing was performed. In light of the apparently isolated cardiac impairment, the patient underwent a Tc99-HMDP bone scintigraphy, in order to differentiate AL from TTR amyloidosis. The test showed an intense fixation of the radiotracers at myocardial level with high heart/whole body uptake ratio (and at the level of the left femur, compatible with known trochanteric fracture) (Figure 2), suggestive of a form related to TTR. In this perspective, the genetic analysis of the TTR gene detected the presence of the Ile68Leu (p.Ile88Leu) mutation.
Figure 1.
Continued.
Figure 1.
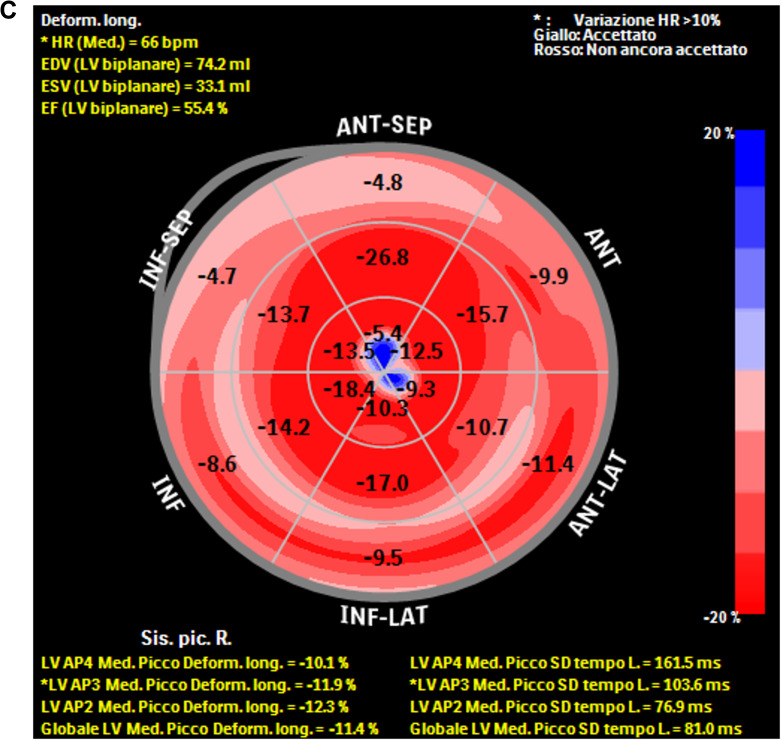
(A) Increased echogenicity of the thickened intraventricular septum, with a speckled appearance (B). The strain of the basal walls is severely reduced in comparison to the apical strain, preserved (C). Improvement in the systolic longitudinal function at follow-up.
Figure 2.
Anterior projection of planar whole-body bone scan, showing an intense cardiac uptake with reduced bone uptake.
The patient was then referred to the Centre for Neuromuscular Diseases of our Hospital, in order to rule-out a concomitant involvement of the peripheral nervous system. The patient reported hypoesthesia in toes and feet associated to walking difficulties for some months; no symptoms of dysautonomia were detected. The neurological examination showed a slight left foot dropping, walking on toes and heels possible only with bilateral support, a reduced excursion in dorsal and plantar flexion of the feet and toes, a mild superficial hypoesthesia from the knees down with absent vibratory sense and an occasional dysesthesia in the feet and hyporeflexia in the lower limbs. A NCS showed an axonal sensory-motor polyneuropathy with associated spontaneous denervation activity. The diagnosis of TTR-FAP stage 1 allowed to initiate a specific therapy with patisiran in February 2021.8 At the follow-up (9 months later), the patient reported less fatigue in daily physical activities with a slight improvement in cardiac ejection fraction (EF 54% vs 45%) and systolic longitudinal function (GL2D-strain −11.4% vs −6.4%) (Figure 1C).
Discussion
In our patient, the presence of a bilateral carpal tunnel syndrome and a specific echocardiographic pattern of cardiac involvement along with a family history of cardiopathy, represented some red flags to suspect a hereditary TTR-amyloidosis. In recent years, the therapeutic landscape of ATTRv greatly expanded, with several molecules targeting the mutated protein. Considering the prognosis and the natural history of the disease,2 it is therefore mandatory not to miss its diagnosis and to address patients to the best available treatment.
A Case of ATTRv Amyloidosis with a Phe64Leu Mutation
A 70-year-old male patient referred sensorimotor disturbances mainly focused in the lower limbs and progressive over two years. In particular, the patient had a bilateral asymmetric distal paresthesia of the lower limbs with a proximal progression associated to hand and feet clumsiness. A concomitant unintended weight loss (15 kg) was also reported. According to the medical history, the patient reported a cured malaria and brucellosis during prolonged working stays in tropical countries, as well as surgery for bilateral stenosing synovitis of third and fourth fingers. His family is originally from Apulia: his mother, deceased due to myocardial infarction, was wheelchair-bound in her seventies, the cause being unknown. He is the second of five siblings: his elder sister had a bilateral carpal tunnel syndrome, and his younger brother was affected by Parkinson’s Disease. The neurological examination showed a slightly ataxic gait with right steppage, a mild weakness of the right foot (MRC 5-/5) and hallux dorsiflexion (4/5), a reduction of the light touch sensation of the first three digits of the hands, the bilateral medial aspects of feet and legs and, to a milder degree, lateral aspects of right foot and leg, a complete loss of vibration sensation at the lower limbs up to the tibial tuberosity but spared joint position sensation. Deep tendon reflexes were diffusely absent. Cranial nerve examination was unremarkable. The NCSs showed a moderate chronic sensorimotor axonal polyneuropathy of the lower limbs together with severe right and moderate left carpal tunnel syndrome according to Padua staging.24 Apart from a moderate hypertrophy of the bilateral peroneal nerve at the fibular head, ultrasound evaluation of the nerve trunks was unremarkable. In particular, the median cross-sectional area at the wrist was within limits, at odds with neurophysiological findings. The patient performed a previous lumbar spine MRI for a traumatic L4 vertebral fracture that, apart from the acute lesion, showed a moderate spinal canal stenosis due to both multiple discal prolapses and hypertrophy of ligamentum flavum. Routine blood chemistries were unremarkable, as well as serology for hepatitis and HIV, protein electrophoresis and immunofixation, an immunological/rheumatological panel and the determination of onconeural, anti-gangliosides, anti-sulfatides and anti-MAG antibodies. Low levels of serum vitamin B12 were treated with parenteral supplementation, without a significant efficacy. The clinical picture of an asymmetric axonal sensorimotor neuropathy associated to a carpal tunnel syndrome and an acquired spinal canal stenosis led to a genetic investigation. After 30 months from symptoms onset, the patient received a diagnosis of FAP associated to the pathogenic Phe64Leu (p.Phe84Leu) mutation of the TTR gene. The same mutation was also observed in three other siblings. A TTR-lowering drug was refused by the patient: for this reason, a treatment with a tetramer stabilizer (tafamidis 20mg per os daily) was started. At that time, the patient was ambulatory using a stick only for longer walks due to initial lower limbs distal involvement, sensory greater than motor. An extended work-up was obtained. Structural MRI imaging showed a diffuse hypertrophy of the lumbosacral plexus and main branching nerves, with a moderate inhomogeneous hyperintensity in T2-weighted sequences. Brachial plexus MRI was unremarkable. The patient had constipation with very rare diarrheic episodes and occasional difficulty in initiating micturition. Parasympathetic function tests were impaired (heart rate variability on deep breathing, Valsalva’s maneuver and upon up-to-standing). Adrenergic sympathetic innervation tests (blood pressure change upon up-to-standing and isometric muscle contraction) and cholinergic sympathetic innervation tests (Sympathetic Skin Reflex and Sudoscan) were normal except for borderline Electrochemical Skin Conductance values at the lower limbs. The patient did not report symptoms of cardiac dysfunction; however, serum NT-proBNP was double the upper limit of normality and a mildly hypertrophic (13 mm) and hyperechogenic septum was observed on transthoracic cardiac ultrasound. However, Tc99-HMDP bone scintigraphy did not show any cardiac uptake, and basal and Holter ECG were within limits. Ocular and renal involvement were also excluded. At the follow-up visit (35 months), he further progressed and required two crutches to move indoor, with an initial distal motor involvement of the upper limbs too. The NCSs showed a mild worsening of the sensorimotor axonal neuropathy, whereas the bilateral carpal tunnel syndrome was stable After an appropriate work-up, tafamidis was withdrawn and inotersen 284 mg weekly by autonomous subcutaneous injection was started. A periodical renal and platelet monitoring was also started.
Four months later, the patient had a rapid weight gain, foamy urines and a significant pitting edema of the four limbs, in absence of respiratory distress. The patient was admitted to our Unit for further investigations, while inotersen was stopped due to a suspect side effect. Cardiac congestive failure and post-renal obstructions were ruled-out, but blood and urine chemistries showed a nephrotic syndrome with non-selective glomerular proteinuria (6.95 g/24h) and preserved renal function. The renal biopsy showed a minimal change glomerulonephritis in absence of amyloid deposits. Screenings for immunological etiologies were inconclusive. Inotersen-related glomerulonephritis is a described albeit rare side effect: for this reason, the drug was withdrawn.25 Water restriction and low doses of amiloride led to the resolution of the acute renal picture in a few days, while proteinuria normalized in the following weeks. Concomitantly, a cardiac MRI showed an infero-basal intramyocardial late enhancement suggestive for cardiac amyloid deposition. 42 months since symptoms onset, when the treatment with patisiran (0.3 mg/kg every three weeks via intravenous infusion) was started, the patient was still ambulatory indoor using crutches and the Norfolk QoL score was 71/156. The drug was well tolerated. Coasting with a further slow decline in neurological condition was observed in the subsequent months, with later stabilization of the clinical picture. Currently, at 58 months since onset, clinical scales show a substantial stability of the severity of the neurological disease (Norfolk QoL score 69/156). Electrochemical Skin Conductance testing showed a positive trend despite stable autonomic symptoms.
Discussion
Patients with a Phe64Leu mutation of the TTR gene usually present with a late-onset sensorimotor neuropathy and intermediate autonomic symptoms and only a late cardiac involvement. Phe64Leu is one of the most frequently observed mutations in patients with ATTRv amyloidosis in Southern Italy,11,14 but it is observed also in other Italian regions due to internal migration. As an indolent, late-onset and often “sporadic” form due to incomplete penetrance, this mutation frequently is responsible for a long diagnostic journey. Amyloid deposits in Phe64Leu ATTRv amyloidosis show low affinity for both Congo red staining26 and Tc99-HMDP bone scintigraphy27 resulting in negative biopsies, a mismatch between structural and scintigraphy imaging and possibly hampering diagnosis and management. Disease-modifying treatments are now widely available for ATTRv amyloidosis, both in the form of tetramer-stabilizing and TTR-lowering drugs,8,25,28 which are able to significantly slow the disease progression after 6 months and are usually well tolerated. A timely clinical and molecular diagnosis and a prompt treatment are thus mandatory to prevent disability and to maintain the quality of life of the patients.
Patisiran for the Treatment of Val30Met ATTRv with Cardiac and Neurological Phenotype
A 78-year-old male patient was admitted to our Amyloid reference centre at Careggi University Hospital, Florence, on January 2018. Eight months before, he developed a progressive weakness in his lower limbs without walking limitation associated to a shortness of breath during physical activity. His family history was negative for cardiovascular or neurological disorders. Past medical history included benign prostatic hyperplasia, urolithiasis, bilateral carpal tunnel syndrome, permanent pacemaker implantation for symptomatic sinus bradycardia, and atrial flutter in chronic anticoagulation therapy. The neurological examination showed a mild broad-based gait, with inability to tandem walk or walk on his toes, and difficulties in walking on his heels. Romberg sign was negative; the left thenar muscle was hypotrophic. The NIS value of muscle weakness (NIS-W) was 19.75: 1 point in finger flexion and thumb abduction on the left, 2 points in ankle dorsiflexor on the right, 3.25 points in ankle dorsiflexor, toe extensor and flexor on the left and 3.5 points in toe extensor and flexor on the right. Reflexes were normal at biceps, triceps and brachioradialis level, and absent at knee and ankle. He complained paresthesias in the bilateral median nerve distribution, hypopallesthesia at distal phalanges of the hands, distal apallesthesia in lower limbs up to his ankles with hypopallesthesia with distal-to-proximal gradient up to his hips, decreased distal pain and light touch sensation in the lower limbs, reduced kinesthetic sensations in the toes. No symptoms or signs of orthostatic hypotension were present. He denied urinary disturbances, or gastrointestinal symptoms except for constipation. The total NIS was 40.75 and the PND score was II. NCS showed markedly reduced compound motor action potential (CMAP) amplitudes in lower limbs (right peroneal nerve: 0.2 mV, left peroneal nerve: 1.4 mV, right tibial nerve: 0.2 mV, left tibial nerve: 1.4 mV) and borderline values in upper limbs (ulnar nerve: 5 mV). SNAPs amplitudes were absent in the lower limbs (sural nerves), absent in the ulnar nerves and markedly reduced in the radial nerves (1.4 microV on the right side and 0.7 microV on the left side). Motor and sensory conduction velocities and electromyography were normal. The transthoracic echocardiography showed an increased wall thickness of both left and right ventricle, with a maximum thickening of 21 mm in the interventricular septum. Left ventricular systolic function was preserved with apical sparing on longitudinal strain and a granular sparkling appearance of the myocardium, bi-atrial enlargement with a restrictive mitral inflow pattern. The Tc99-HMDP bone scintigraphy showed a heart uptake with a Perugini visual score 2. Serum and urinary immunofixation electrophoresis excluded the presence of monoclonal components and the serum free light chain assay showed a normal κ/λ ratio. An ATTRv amyloidosis was suspected: the genetic test for the TTR gene found a Val30Met (p.Val50Met) mutation of the TTR gene. Therefore, on December 2018 tafamidis 20 mg daily was started.29,30 Despite the treatment with tafamidis, the walking impairment progressed and the patient needed a crutch to walk due to postural instability and worsening of the weakness in lower limbs; the cardiac involvement remained stable. On December 2019, the neurological examination showed an ataxic gait with marked instability and a positive Romberg sign; the strength in distal muscles of the upper and lower limbs decreased, and the NIS-W score was 33.25. Reflexes impaired in upper limbs, with absence of the biceps and triceps and reduction of the brachioradialis; he started to complain paresthesias in feet too. Besides, a worsening of vibratory sensation in upper limbs with hypopallesthesia up to his elbows and of proprioception with akinesthesia in the toes was observed. The total NIS score was 65.25 and the PND score was IIIa. On January 2020, due to clinical worsening, a treatment with patisiran 0.3 mg/kg iv every 3 weeks was started.8 No adverse events occurred and the therapy was well tolerated. After almost 2 years since the beginning of patisiran, the walking disability of the patients remained stable (PND IIIa), as well as the sensitive deficits; the neurological examination showed a slight improvement in strength in some distal muscles of upper and lower limbs; the NIS-W score as 32 and the total NIS score was 64. The transthoracic echocardiography showed a slight reduction of wall thickening of interventricular septum (19 mm vs 21 mm) and a stability of left ventricular systolic function and atrial enlargement. Besides, a progressive reduction of NTproBNP values was reported: from 3419 ng/L (normal value < 125 ng/L) on December 2020 to 1488 ng/L on July 2021.
Discussion
In our tafamidis refractory patient, the treatment with patisiran was able to halt the progression of the neuropathy and to induce a slight improvement in cardiac parameters without any appreciable toxicity. Although the single case report provides only anecdotal evidence to support the efficacy of patisiran in this kind of patient, the observed results, together with data from some recent trials8,9 could change the treatment scenario for cardiac and neuropathic ATTRv amyloidosis.
ATTRv with a Pathogenic Val122Ile Heterozygous Mutation
A 76-year-old male patient suffered in 2016 from a sick sinus syndrome with paroxysmal atrial fibrillation treated with catheter ablation in 2018 and subsequent implantation of a permanent pacemaker. His past medical history was positive for right hemicolectomy due to a colon cancer in 2009, mild hypertension and stage I chronic kidney disease (serum creatinine 16 mg/L; asymptomatic proteinuria 150 mg/die). He had no family history for heart disease or neuropathy in his first-degree relatives. Starting from 2017, he also reported a weight loss (5 Kg over 1 year), and from the end of 2018 a progressive worsening of dyspnea and severe asthenia without pain. Heart failure was diagnosed through an increase of NT-pro BNP (3553 ng/L) and troponin T (82 ng/L) levels, without any ST-T change on ECG. Thyroid stimulating hormone, folic acid, B12 levels and hemoglobin A1c values were normal, and no monoclonal protein was present on serum or urine immunofixation. Vital signs were the following: blood pressure 110/70 mmHg, heart rate 65 bpm, body mass index 1.8 kg/m2, oxygen saturation at rest 96% on room air. The cardiac auscultation showed a regular rhythm soft 2/6 holosystolic murmur at the left lower sternal border; no wheezing, rales or rhonchi in his lungs, as well as no hepatosplenomegaly or leg swelling were observed. On initial evaluation, his symptoms were consistent with New York Heart Association (NYHA) functional class III. The ECG parameters were the following: sinus rhythm, heart rate of 65 beats/min, normal axis, P waves induced by pacemaker, low voltage of QRS complex and no ST-segment changes. The transthoracic echocardiogram was suggestive of infiltrative cardiomyopathy, showing a nondilated left ventricle (EDV 100 mL - DTD 46 mm) with preserved ejection fraction (55%), interventricular septum of 18 mm and posterior wall of 13 mm; grade 2 diastolic dysfunction (E/A 2.5; E/e’ avg 11); severe bi-atrial dilatation (LA 58 mL/mq, RA 20 cmq), moderate mitral regurgitation (rPISA 8 mm; ERA 0.28 cmq; RV 47 mL), moderate tricuspidal regurgitation (rPISA 5 mm) with VA gradient of 60 mmHg and PAPs 65 mmHg. The global longitudinal strain (LS) showed the typical pattern of “relative apical sparing”, with a reduction in LS in the basal and mid-myocardial segments, with relative sparing of the left ventricular apex. Tc−99Mpyrophosphate (PYP) single-photon-emission computed tomography was Perugini grade 2, and immune-histochemical test at labial salivary glands biopsy confirmed the diagnosis of ATTRv amyloidosis. The mental and cranial nerve tests were normal; the 6MWT value was below 100 m; strength testing was grade 5 according to the Medical Research Council scale for all muscles tested except for toe extensors and flexors (grade 4), with a mild postural tremor in the upper limbs; reflexes in the lower limbs were absent; thermal, pin-prick and touch pressure sensation were normal, but vibration and joint position sensation were decreased on great toe. Symptoms or dysautonomic signs were not found and Romberg sign was negative. Electroneurography was normal in upper limbs, showing mild slowed conduction velocities in peroneal and tibial nerves (37–39 m/s), a moderate decreased compound muscle action potential amplitude at flexor hallucis brevis and extensor digitorum brevis (1, 1–1, 8 mV), and a decreased antidromic sensory nerve action potential amplitude and velocity of sural nerves (respectively 2–4 μV and 36–38 m/s). Distal motor latencies were in the normal range, without any conduction block; the sympathetic skin response showed preserved postganglionic sudomotor components. The needle electromyographic test was normal in upper limb and proximal lower limb muscles, but showing large, long, and sometimes polyphasic motor units in extensor digitorum brevis and extensor hallucis longus with reduced recruitment but without spontaneous activity. Overall, the neurophysiological study showed a mild predominant-axonal sensory-motor length-dependent polyneuropathy. TTR genetic test showed a pathogenic heterozygous mutation Val122Ile (p.Val142Ile) consistent with diagnosis of ATTRv amyloidosis. As the criteria for prescribing small interfering RNA (siRNA) therapy were met (ATTRv in patients with stage 1 polyneuropathy), a treatment with patisiran 300 μg/Kg body weight iv every 3 weeks was started (October 2020). At baseline, the NIS and Norfolk QoL-DN scores were 12 and 25, respectively. The day after the first siRNA administration, the patient complained a worsening of dyspnea and an increased NT-proBNP value (from 4269 to 9922 ng/L), without any significant change on ECG and troponin level, returned at baseline status within the 48 hours. The dyspnea improved from the third day after the first infusion of patisiran: for this reason, the three-weekly therapy was confirmed. A significant improvement on echocardiographic parameters (grade I diastolic function with E/e’ avg 9, mild mitral regurgitation with estimated PAPs 45 mmHg) was observed after approximately 6 months from the beginning of the therapy. At the one-year follow-up, no other side effects were reported and asthenia, dyspnea, Norfolk QoL-DN scale (14) and 6MWT (over 250 meters) significantly improved, without any significant change on NIS scale (11) and neurophysiological parameters. Besides, respect to baseline, after 1 year serum concentration of TTR (from 340 to 70 mg/L), creatinine (from 16 to 13 mg/L) and albumin (from 42 g/L to 38 g/L) decreased respect to baseline; troponin T and NT-pro BNP levels were unchanged until the last evaluation (October 2021), when a significant increase occurred (respectively 6723 ng/L and 3069 ng/L), without dyspnea and relevant changes of EKG and echocardiographic parameters.
Conclusion
This paper underlines the impact of patisiran in 9 patients affected by ATTRv amyloidosis from non-endemic areas in Italy. Many patients received a wrong diagnosis with significant diagnostic delay. TTR gene testing can be used to confirm ATTRv amyloidosis and should be performed early if the condition is suspected.2,31 Hence, ATTRv amyloidosis should be early suspected to achieve the best treatment. Also, the management of patients with ATTRv amyloidosis needs a multidisciplinary approach. The therapeutic strategy for ATTRv amyloidosis is now widen than in the recent past, including, other than liver transplantation, TTR stabilizers and TTR gene silencers. Most patients benefit from these active drugs regardless of the stage of their disease and the involved mutation.1,2
In particular, patisiran is a small, double-stranded interfering RNA encapsulated in a lipid nanoparticle, able to penetrate into hepatocytes, where it selectively targets TTR mRNA, reducing TTR production.7 The phase 3 APOLLO confirmed the efficacy and safety of patisiran in patients with ATTRv amyloidosis, halting or regressing the disease progression in some cases.8 Recent studies have correlated improved functional and biochemical outcomes with a regression of amyloid burden, especially at the cardiac level.32 Literature data, as well as the above presented case reports, show that this drug is effective and safe in improving both neurological and cardiovascular symptoms of ATTRv amyloidosis, and to maintain a good QoL, independently form the stage of the disease and the involved mutation. Its efficacy and safety were confirmed for the long-term use too. Today, patisiran can be considered a valid therapeutic option for the management of patients with ATTRv amyloidosis and polyneuropathy and cardiovascular symptoms.
Acknowledgments
Substantial intellectual contribution: Novo G and Di Lisi D (Department of Internal Medicine and Specialties, University of Palermo, Palermo); Brighina F (Department of Biomedicine, Neuroscience and advanced Diagnostic, University of Palermo, Palermo); Filosto M (NeMO-Neuromuscular OminCenter-Brescia Clinical Center for Neuromuscular Diseases, Brescia); Padovani A, Risi B (Department of Clinical and Experimental Sciences, University of Brescia, Brescia), Nardi M, Tomasoni D, Bonelli A (Cardiology Unit, Spedali Civili and University of Brescia), Faggiano P (Fondazione PoliAmbulanza Brescia), Cappelli F, Allinovi M (Tuscan Regional Amyloidosis Centre, Careggi University Hospital, Florence), Casagrande S (Tuscan Regional Amyloidosis Centre, Careggi University Hospital, Florence; Neurosciences Department, Florence University), Barilaro A (Tuscan Regional Amyloidosis Centre, Careggi University Hospital, Florence; Neurosciences Department, Careggi University Hospital, Florence, Italy), Stelitano M, Russo C (Division of Cardiology, Heart Vascular and Thoracic Department, Città della Salute e della Scienza (Molinette Hospital- University of Turin), Turin, Italy).
Funding Statement
Alnylam Italia gave an unconditional support for the publication of case reports of patients with ATTRv amyloidosis treated with patisiran.
Disclosure
Fanara S, Fava A, Ferrero B, Gentile L, Giorgi M, Perfetto F, Poli L, Russo M, Russo D, Vastola M declare no conflicts of interest in this work. Di Stefano V received congress and travel accommodation expense compensations from Alnylam. Guaraldi P has been advisory board member of Alnylam; received speaker fees and honoraria from Theravance Biopharma, Akcea Therapeutics and Chiesi; received congress and travel accommodation expense compensations from Alnylam, Bial, Zambon, AbbVie and Sobi, non-financial support from Pfizer. Leonardi L received travel grants from Swedish Orphan Biovitrum AB (SOBI) and speech/board grants from Alnylam Pharmaceutical. Tagliapietra M received travel grants from Alnylam and Sobi and a training grant sponsored by Pfizer.
References
- 1.Russo M, Gentile L, Di Stefano V, et al. Use of drugs for ATTR amyloidosis in the real world: how therapy is changing survival in a non-endemic area. Brain Sci. 2021;11(5):545. doi: 10.3390/brainsci11050545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams D, Koike H, Slama M, Coelho T. Hereditary transthyretin amyloidosis: a model of medical progress for a fatal disease. Nat Rev Neurol. 2019;15(7):387–404. doi: 10.1038/s41582-019-0210-4 [DOI] [PubMed] [Google Scholar]
- 3.Conceicao I, González-Duarte A, Obici L, et al. “Red- flag” symptom clusters in transthyretin familial amyloid polyneuropathy. J Peripher Nerv Syst. 2016;21:5–9. doi: 10.1111/jns.12153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magliano L, Obici L, Sforzini C, et al. Psychosocial burden and professional and social support in patients with hereditary transthyretin amyloidosis (ATTRv) and their relatives in Italy. Orphanet J Rare Dis. 2021;16:163. doi: 10.1186/s13023-021-01812-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koike H, Okumura T, Murohara T, Katsuno M. Multidisciplinary approaches for transthyretin amyloidosis. Cardiol Ther. 2021;10(2):289–311. doi: 10.1007/s40119-021-00222-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russo M, Gentile L, Toscano A, Hammed Aguennouz M, Vita G, Mazzeo A. Advances in treatment of ATTRv amyloidosis: state of the art and future prospects. Brain Sci. 2020;10:952. doi: 10.3390/brainsci10120952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suhr OB, Coelho T, Buades J, et al. Efficacy and safety of patisiran for familial amyloidotic polyneuropathy: a Phase II multi-dose study. Orphanet J Rare Dis. 2015;10:109. doi: 10.1186/s13023-015-0326-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams D, Gonzalez-Duarte A, O’Riordan WD, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):11–21. doi: 10.1056/NEJMoa1716153 [DOI] [PubMed] [Google Scholar]
- 9.Solomon SD, Adams D, Kristen A, et al. Effects of patisiran, an RNA interference therapeutic, on cardiac parameters in patients with hereditary transthyretin-mediated amyloidosis. Circulation. 2019;139(4):431–443. doi: 10.1161/CIRCULATIONAHA.118.035831 [DOI] [PubMed] [Google Scholar]
- 10.Adams D, Polydefkis M, González-Duarte A, et al. Long-term safety and efficacy of patisiran for hereditary transthyretin-mediated amyloidosis with polyneuropathy: 12-month results of an open-label extension study. Lancet Neurol. 2021;20(1):49–59. doi: 10.1016/S1474-4422(20)30368-9 [DOI] [PubMed] [Google Scholar]
- 11.Mazzeo A, Russo M, Di Bella G, et al. Transthyretin-Related Familial Amyloid Polyneuropathy (TTR-FAP): a single-center experience in Sicily, an Italian endemic area. J Neuromuscul Dis. 2015;2(s2):S39–48. doi: 10.3233/JND-150091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solé G, Salort-Campana E, Pereon Y, et al. Guidance for the care of neuromuscular patients during the COVID-19 pandemic outbreak from the French rare health care for neuromuscular diseases network. Rev Neurol. 2020;176(6):507–515. doi: 10.1016/j.neurol.2020.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Stefano V, Battaglia G, Giustino V, et al. Significant reduction of physical activity in patients with neuromuscular disease during COVID-19 pandemic: the long-term consequences of quarantine. J Neurol. 2021;268(1):20–26. doi: 10.1007/s00415-020-10064-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russo M, Obici L, Bartolomei I, et al. ATTRv amyloidosis Italian registry: clinical and epidemiological data. Amyloid. 2020;27(4):259–265. doi: 10.1080/13506129.2020.1794807 [DOI] [PubMed] [Google Scholar]
- 15.Gentile L, Russo M, Luigetti M, et al. Patisiran in hATTR amyloidosis: six-month latency period before efficacy. Brain Sci. 2021;11(4):515. doi: 10.3390/brainsci11040515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perugini E, Guidalotti PL, Salvi F, et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3, 3-diphosphono-1, 2-propanodicarboxylic acid scintigraphy. J Am Coll Cardiol. 2005;46(6):1076–1084. doi: 10.1016/j.jacc.2005.05.073 [DOI] [PubMed] [Google Scholar]
- 17.Dyck PJ, Sherman WR, Hallcher LM, et al. Human diabetic endoneurial sorbitol, fructose, and myo-inositol related to sural nerve morphometry. Ann Neurol. 1980;8:590–596. doi: 10.1002/ana.410080608 [DOI] [PubMed] [Google Scholar]
- 18.Mayaudon H, Miloche PO, Bauduceau B. A new simple method for assessing sudomotor function: relevance in type 2 diabetes. Diabetes Metab. 2010;36:450–454. doi: 10.1016/j.diabet.2010.05.004 [DOI] [PubMed] [Google Scholar]
- 19.Lauria G, Hsieh ST, Johansson O, et al. European federation of neurological societies/peripheral nerve society guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol. 2010;17(903–12):e44–9. [DOI] [PubMed] [Google Scholar]
- 20.Gentile L, Tournev I, Amass L, Chapman D, Mazzeo A; THAOS investigators. Phenotypic differences of Glu89Gln genotype in ATTR amyloidosis from endemic loci: update from THAOS. Cardiol Ther. 2021;10:481–490. doi: 10.1007/s40119-021-00226-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berk JL, Suhr OB, Obici L, et al. Repurposing diflunisal for familial amyloid polyneuropathy: a randomized clinical trial. JAMA. 2013;310:2658–2667. doi: 10.1001/jama.2013.283815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.González-Duarte A, Berk JL, Quan D, et al. Analysis of autonomic outcomes in APOLLO, a phase III trial of the RNAi therapeutic patisiran in patients with hereditary transthyretin-mediated amyloidosis. J Neurol. 2020;267(3):703–712. doi: 10.1007/s00415-019-09602-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saito M, Nakao Y, Higaki R, et al. Clinical significance of the relative apical sparing pattern of longitudinal strain in patients with cardiac amyloidosis. Eur Heart J. 2020;41(2):2012. doi: 10.1093/ehjci/ehaa946.101232101604 [DOI] [Google Scholar]
- 24.Padua L, LoMonaco M, Gregori B, Valente EM, Padua R, Tonali P. Neurophysiological classification and sensitivity in 500 carpal tunnel syndrome hands. Acta Neurol Scand. 1997;96:211–217. doi: 10.1111/j.1600-0404.1997.tb00271.x [DOI] [PubMed] [Google Scholar]
- 25.Benson MD, Waddington-Cruz M, Berk JL, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med. 2018;379:22–31. doi: 10.1056/NEJMoa1716793 [DOI] [PubMed] [Google Scholar]
- 26.Luigetti M, Romozzi M, Bisogni G, et al. hATTR pathology: nerve biopsy results from Italian referral centers. Brain Sci. 2020;10:780. doi: 10.3390/brainsci10110780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Musumeci MB, Cappelli F, Russo D, et al. Low sensitivity of bone scintigraphy in detecting Phe64Leu mutation-related transthyretin cardiac amyloidosis. JACC Cardiovasc Imaging. 2020;13:1314–1321. doi: 10.1016/j.jcmg.2019.10.015 [DOI] [PubMed] [Google Scholar]
- 28.Coelho T, Maia LF, Martins `da Silva A, et al. Tafamidis for transthyretin familial amyloid polyneuropathy: a randomized, controlled trial. Neurology. 2012;79:785–792. doi: 10.1212/WNL.0b013e3182661eb1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coelho T. Tafamidis: a novel and effective oral treatment for familial amyloid neuropathies. Eur J Neurol. 2012;19(Suppl 1):8. [Google Scholar]
- 30.Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007–1016. doi: 10.1056/NEJMoa1805689 [DOI] [PubMed] [Google Scholar]
- 31.Adams D, Algalarrondo V, Polydefkis M, Sarswat N, Slama MS, Nativi-Nicolau J. Expert opinion on monitoring symptomatic hereditary transthyretin-mediated amyloidosis and assessment of disease progression. Orphanet J Rare Dis. 2021;16(1):411. doi: 10.1186/s13023-021-01960-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fontana M, Martinez-Naharro A, Chacko L, et al. Reduction in CMR derived extracellular volume with patisiran indicates cardiac amyloid regression. JACC Cardiovasc Imaging. 2021;14(1):189–199. doi: 10.1016/j.jcmg.2020.07.043 [DOI] [PubMed] [Google Scholar]