Abstract
Although there is an ever-increasing number of disease-modifying treatments for relapsing multiple sclerosis (MS), few appear to influence coronavirus disease 2019 (COVID-19) severity. There is concern about the use of anti-CD20-depleting monoclonal antibodies, due to the apparent increased risk of severe disease following severe acute respiratory syndrome corona virus two (SARS-CoV-2) infection and inhibition of protective anti-COVID-19 vaccine responses. These antibodies are given as maintenance infusions/injections and cause persistent depletion of CD20+ B cells, notably memory B-cell populations that may be instrumental in the control of relapsing MS. However, they also continuously deplete immature and mature/naïve B cells that form the precursors for infection-protective antibody responses, thus blunting vaccine responses. Seroconversion and maintained SARS-CoV-2 neutralizing antibody levels provide protection from COVID-19. However, it is evident that poor seroconversion occurs in the majority of individuals following initial and booster COVID-19 vaccinations, based on standard 6 monthly dosing intervals. Seroconversion may be optimized in the anti-CD20-treated population by vaccinating prior to treatment onset or using extended/delayed interval dosing (3–6 month extension to dosing interval) in those established on therapy, with B-cell monitoring until (1–3%) B-cell repopulation occurs prior to vaccination. Some people will take more than a year to replete and therefore protection may depend on either the vaccine-induced T-cell responses that typically occur or may require prophylactic, or rapid post-infection therapeutic, antibody or small-molecule antiviral treatment to optimize protection against COVID-19. Further studies are warranted to demonstrate the safety and efficacy of such approaches and whether or not immunity wanes prematurely as has been observed in the other populations.
Keywords: autoimmunity, CD20 B cells, COVID-19 vaccination, immunotherapy, multiple sclerosis
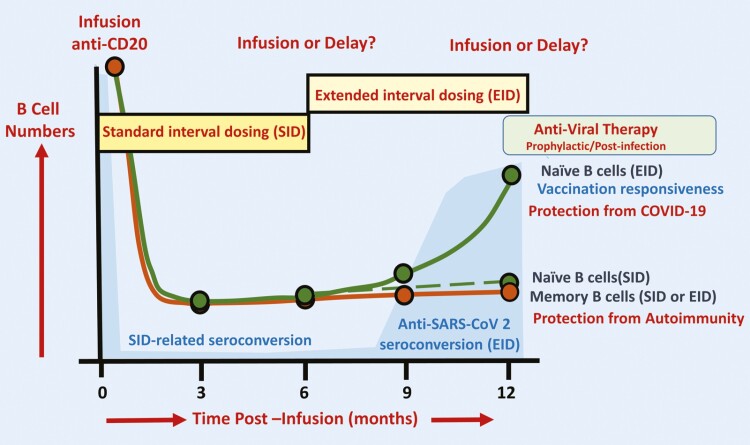
We review emerging evidence indicating that differential rates of B-cell subset repopulation following CD20-depleting antibody treatments can be exploited, to maintain control of autoimmunity whilst allowing SARS-CoV-2 vaccination-induced seroconversion. Extended-interval antibody dosing, supported by use of antiviral antibodies or small molecules may help optimize protection against severe COVID-19.
Graphical Abstract
Graphical Abstract.
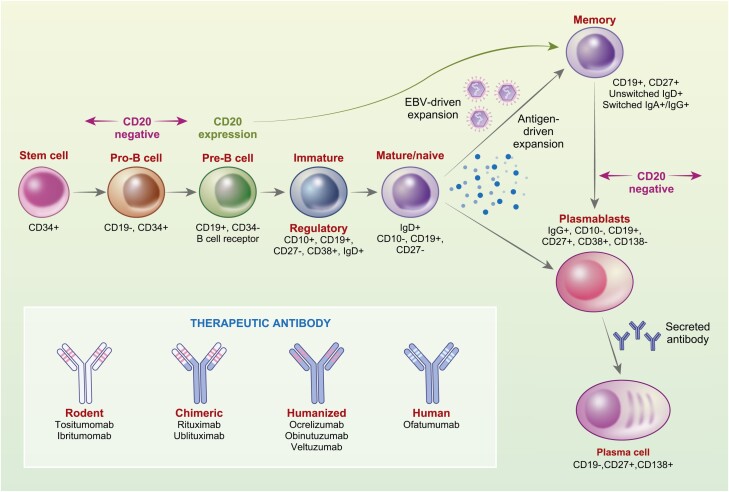
Therapeutic B-cell-targeting antibodies are used in the treatment of autoimmune diseases; most recently as a maintenance treatment for the control of multiple sclerosis (MS). Membrane-spanning 4A1 (CD20) protein is a cell membrane molecule that is involved in the development and differentiation of B cells and is thought to represent a type of calcium channel [1]. It is expressed throughout B-cell development except in early (stem cells and pro-/pre-B cells) and late (plasmablasts and plasma cells) stages (Fig. 1) [1]. B cells can be targeted by an increasing number and variety of CD20-depleting monoclonal antibodies (mAb) including: murine (Tositumomab and ibritumomab used as radioactive isotope targeting vehicles for lymphoma); chimeric (rituximab used in lymphoma, leukaemia, rheumatoid arthritis, vasculitis, pemphigus vulgaris and used off-label in many other autoimmune diseases including MS and ublituximab for MS); humanized (ocrelizumab for MS; obinutuzumab for B-cell lymphomas and leukaemia; veltuzumab for idiopathic thrombocytopaenic purpura and pemphigus), and human (ofatumumab used intravenously from chronic lymphocytic leukaemia and subcutaneously for MS) antibodies (Fig. 1) [2]. These cause complement-dependent killing, antibody-dependent cellular cytotoxicity, and apoptosis of CD20 expressing B cells [3]. In autoimmune disease, efficacy may relate to either the direct long-term depletion of memory B cells (Fig. 2) and development of regulatory B cells within the regenerating CD19 population [4–6] or indirectly through blockade of T-cell activity to inhibit autoimmunity [7, 8] (Fig. 1).
Figure 1:
B-cell lineage and CD20-specific antibodies. A simplified schematic of the B-cell lineage related to CD20 antigen expression and the CD20-specific antibodies. Epstein–Barr virus (EBV) can generate memory B cells in the potential absence of antigen and co-stimulation or they can be antigen expanded.
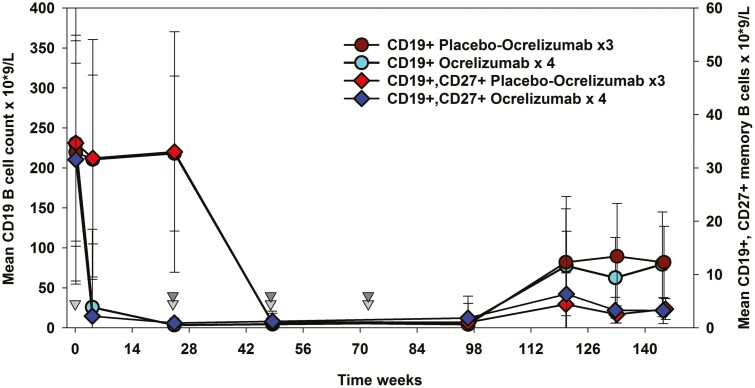
Figure 2:
Deletion and repopulation of B cells following ocrelizumab infusion in MS. Individuals received 600 mg ocrelizumab Q24W for four cycles or placebo followed by three 600 mg ocrelizumab cycles [6]. The raw data were extracted from the phase II ocrelizumab extension study supplied via the www.vivli.org portal using R software. The results represent the mean ± standard deviation; n = maximum 46–47/group.
CD20-depletion is a risk factor for severe symptomatic COVID-19
Coronavirus disease 2019 (COVID-19) has been a devastating global pandemic, killing millions of people. Although the major drivers of disease severity relate to age, sex, comorbidities, socioeconomic factors, and viral load [9, 10] there is concern that disability and being immunocompromised may contribute to COVID-19 disease morbidity in the MS population [11–13]. CD20-depleting mAb have been reported to increase hospitalization and more severe COVID-19 in many [13–19], but not all [20, 21], studies in MS. As anti-CD20 therapies appear to limit antibody responses [22–24], this apparent increased risk from COVID-19 infection [13] may relate to the inability to form, or loss of, a protective, cross-reactive immunity to cold-causing coronavirus responses [25–28]. The ability of specific antibody responses to inhibit severe acute respiratory syndrome corona virus two (SARS-CoV-2) infection in animal models [29, 30] and humans [31–34] highlights the importance of the B-cell response for protection. A sufficiently high neutralizing titre may limit symptomatic SARS-CoV-2 infection [35, 36] more than a T-cell response [37]. However, a B-cell response is not absolutely necessary, as the SARS-CoV-2 virus can be eliminated by the innate-immune and T-cell response before the formation of an effective IgG response, and recovery can occur in the relative absence of B cells [23, 25]. However, the full spectrum of innate, T- and B-cell immunity will provide the best protection [38].
CD20-depletion is also a risk factor for poor serological response to infection and vaccination
Given the blunted antibody response to other vaccines [23, 39, 40], it is not surprising that CD20-depleting antibodies, notably rituximab and ocrelizumab, have been repeatedly and consistently shown to induce poor seroconversion following natural infection with SARS-CoV-2 [41–46]. Likewise, although RNA vaccines produce higher antibody titres and result in greater proportional seroconversion than adenoviral vector vaccines [24, 35, 47, 48], seroconversion in CD20-depleted, COVID-19-vaccinated individuals is universally poor [22, 24, 49–55]. It is evident that it is possible to generate a COVID-19 vaccine response in the absence of detectable peripheral B cells [55–57], indicating that the generation of the vaccine-related antibody response likely occurs within lymphoid tissues, which are seemingly not completely purged of B cells [5], rather than the peripheral blood. However, baseline B-cell number within the blood has potential biomarker activity for predicting seroconversion following vaccination [58–61]. People with 1–3% CD19/>10 cells/µl, often, but not always, generate COVID-19-related IgG responses following vaccination [50, 56, 60–62] related to repopulation of naïve B cells. Depletion with CD38-specific antibodies, as used in myeloma, can also be associated with poor seroconversion [63, 64] supporting a role for CD20+ naïve B, although CD38 is also found on CD20−, plasmablasts, and plasma cells (Fig. 1).
Despite a consistently blunted antibody response in those treated with anti-CD20 mAb, it is increasingly clear that T-cell responses are often generated following both natural infection and COVID-19 vaccination [22, 41, 50, 53, 57, 65–67]. CD4 responses may not only facilitate antibody responses, but can also provide help for other defence mechanisms against the SARS-CoV-2 virus that are augmented by vaccination [25, 50, 57, 65–67]. CD8 responses may even be augmented in antibody-deficient individuals in MS and elsewhere [50, 59, 68], perhaps consistent with mobilization of CD8 T cells by vaccination [69]. Such viral spike protein directed CD8 responses from vaccination [48], may complement protective CD8 responses to other viral proteins, such as the nucleocapsid protein that are generated following natural infection with SARS-CoV-2 or in some instances with other coronaviruses [25, 70, 71].
However, given the importance of neutralizing antibody responses following vaccination [37, 38], and the finding that protective antibody titres subside over time [35], COVID-19 breakthrough can and will occur. This is already seen in vaccinated, healthy individuals [72–75] and is being seen in immunosuppressed individuals [76, 77]. As CD20-treated individuals produce lower titre antibody responses than untreated controls [24, 49, 78], they are potentially in need of effective third cycle/booster vaccinations. Whilst boosters increase seroconversion in some immunocompromised people [79, 80], it is likely that CD20-depletion will still inhibit this response in the majority of people, as is currently being seen [80–83] There is thus a potential need for pilot studies to help optimize COVID-19 vaccination in the anti-CD20-treated population before mass use of a potentially futile strategy.
Long-term memory B-cell depletion may support safe treatment breaks for vaccination
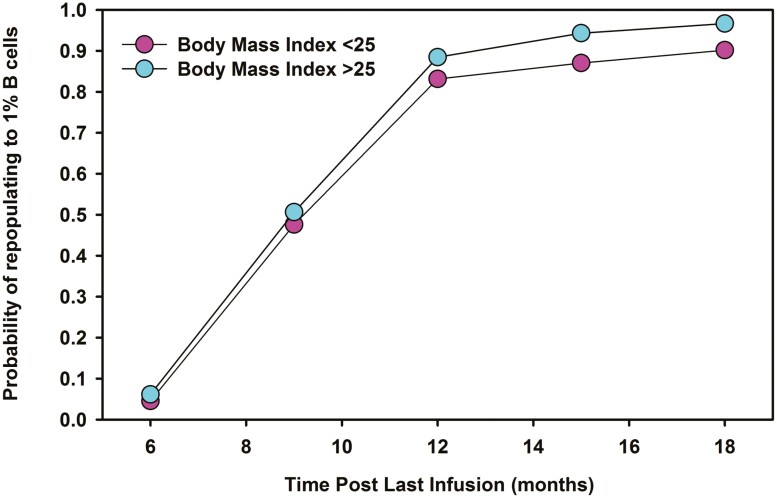
The inhibition of vaccine-induced antibody responses by continuous CD20-depletion [23, 39, 84] is not surprising, as B cells repopulate in a stereotyped behaviour following depletion with CD20-depleting mAb [5, 6, 23, 85]. Immature/transitional/regulatory B cells (Fig. 1) rapidly repopulate the space created by B-cell depletion and generate a novel mature/naïve B-cell pool containing cells that can respond to new antigenic stimuli to potentially generate vaccine responses [5, 23, 85]. Following 600 mg 24QW doses of ocrelizumab, it takes on average 62–72 weeks (range 27–175 weeks) for CD19 cells to return to the lower limit of normal (80 cells/μl) [6, 23]. Repopulation following 500 mg/1000 mg 24QW rituximab administration is more rapid [85, 86]. Depletion of CD19+ cells following 20 mg subcutaneous ofatumumab injections is rapid and sustained during treatment [87]. Following treatment cessation, it takes a median of about 25 weeks for CD19+ cells to repopulate to 40 cells/μl, which is faster than found with repeated doses of ocrelizumab [88, 89]. The degree of depletion and speed of repopulation induced by ocrelizumab may depend on both the dose used in vivo and the individual, most notably related to body mass index (BMI), where larger people may repopulate quicker [90–93].
In contrast, the memory B-cell pool, which potentially harbours important pathogenic response cells, repopulates very slowly over many months [4, 5, 94] (Fig. 2). This may provide durability of protection against autoimmunity [4, 5]. The majority of people do not show disease reactivation within 12 to 18 months following treatment cessation following rituximab and ocrelizumab treatment [6, 95, 96] Following the development of the COVID-19 pandemic, concerns about the influence of immunosuppression led to treatment interruption [25]. Delays of 1–3 and even 6 months were not generally associated with disease breakthrough [96–102].
Is it possible to optimize vaccine response through treatment delay?
There is increasing evidence that antibody responses relate to the degree of B-cell depletion and repopulation [56, 62, 103, 104]. However, B-cell repletion to 1% CD19+ lymphocytes occurred in less than 5% of people at 6 months following 3–4 cycles of ocrelizumab (Fig. 3). Therefore, a large population of people established on treatment are unlikely to be able to mount an effective COVID-19 vaccine antibody response within the 6-month dosing schedule [53, 105]. However, about 85–90% of people exhibited a 1% CD19+ B-cell level at 12 months following ocrelizumab [106]. It was evident that even at 18 months post-infusion some people had not repleted to 1% B cells (Fig. 3). A higher BMI (>25) may exhibit a small influence on B-cell depletion and repopulation (Fig. 3) [93, 107]. This could argue for a more personalized dosing regime as is currently employed with off-label rituximab in MS and a number of other autoimmune diseases, allowing >6 monthly extended dosing intervals based on B-cell repletion [6, 108, 109]. It is evident that people are willing to accept delays in ocrelizumab and rituximab treatment [96–102]; therefore, offering an extended dosing interval with CD20-depleting mAb infusions is feasible and may safely allow better seroconversion responses for the majority of people [53, 96, 106]. Given the novelty of monthly ofatumumab injections, vaccination prior to treatment onset should be feasible for most people. How this agent will influence future COVID-19-related and other vaccinations, and the safety of treatment delays, is currently unknown. Therefore, is not possible to offer evidence-based advice to assist patient choice for this treatment.
Figure 3:
CD19 B-cell repletion after repeated ocrelizumab infusions. Individuals received 600 mg ocrelizumab Q24W for three or four cycles followed an 18-month treatment-free period [6]. The data were extracted the raw data from the phase II ocrelizumab extension study (NCT00676715) supplied via the Vivli Inc. portal using R software. Data were stratified according to baseline body mass index. The results represent the approximate time from the last infusion and probability of repopulating to 1% CD19 B lymphocytes.
Generating a protective antibody response
An alternative solution to extended dosing or boosters may be to provide a prophylactic antiviral response through the use of small-molecule antiviral agents, such as Molnupiravir, which are in development [110–112], or the generation of a high-titre antibody response through the delivery of convalescent sera or mAb cocktails that can be optimized for activity against circulating variants [112–115]. Intravenous or subcutaneous SARS-CoV-2 mAb cocktails such as casirivimab/imdevimab and bamlanivimab/etesevimab [32, 33], against different parts of the SARS-CoV-2 spike protein may offer the potential to provide prophylactic treatment in people who are not expected to mount an adequate immune response to complete SARS-CoV-2 vaccination [32, 112]. These have shown some protection in CD20-depleted individuals [113, 116]. However, their benefit will depend on efficacy against SARS-CoV-2 variants of concern circulating with the population at the time of use [117, 118]. Some of these agents have standard antibody half-lives and require frequent administration, perhaps limiting their long-term use [112]. However, they have potential for targeted prophylaxis, such as following exposure to household infection [32]. Long-acting antibodies that are Fc-manipulated to substantially increase serum half-lives, such as a tixagevimab/cilgavimab cocktail [114, 115], may offer more widespread benefit (Table 1).
Table 1:
Prophylactic inhibition of COVID-19 infection
| Demographic | Treatment | Protection | ||
|---|---|---|---|---|
| Baseline subgroup | Onset of case | AZD7442 | Placebo | Relative risk reduction |
| All participants | All cases | 23/749 | 17/372 | 33% (−26 to 65) reduction |
| PCR-negative | All cases | 6/715 | 11/358 | 73% (27 to 90) reduction |
| PCR-negative | 7 days | 1/170 | 6/352 | 92% (32 to 99) reduction |
Participants (adults > 18 years old) with a potential exposure to an affected individual were 1:2 randomized to saline placebo (n = 372) or a single set of intramuscular 300 mg tixagevimab/cilgavimab [(AZD7442) n = 749] in a double-blind, randomized trial (STORMCHASER; NCT04625972). Whilst the primary endpoint, triggered after 35 infection events, of illness occurring up to day 183 post-potential contact, was not met, unplanned post hoc analysis of individuals who were confirmed viral polymerase chain reaction (PCR) test negative at the start of the trial and did not develop disease for 7 days after infusion, to avoid analysis of people infected before infusion, showed marked prophylactic protection [115].
However, a concern is that untreated, immunosuppressed individuals may harbour prolonged SARS-CoV-2 infection that could allow serial mutations to develop, impacting on infectivity and immune escape [119–122]. This view is tempered by the alternative possibility that evolution of the virus selected by the presence of convalescent sera/mAb cocktails could drive the selection of viral escape mutants [123]. Whilst mAb cocktails have been developed to limit this risk [32, 33, 112], this remains a potential problem.
Conclusions
At initial vaccine roll-out, the priority was to vaccinate all people with MS in the timeliest manner possible, to provide some immunity against COVID-19 [124]. However, recent data enable us to take a more considered approach on the best way to balance protecting people taking CD20-depleting antibodies, whilst maintaining effective disease control. Although blunted, many people make some form of response; a simple approach would be to determine whether boosters can augment this, as suggested by early evidence [77, 83]. A growing body of evidence appears to show that inactivated and adenoviral-based vaccines generate lower titre antibody responses and potentially weaker protection than RNA vaccines [24, 35, 48]; thus, booster injections should ideally focus on RNA vaccines, where mRNA-1273 appears to give the highest titre response [35, 52]. In some places, it may be feasible to offer whole inactivated virus vaccines and whilst they may not offer comparable protection from infection to RNA vaccines [48], they expose the immune response, notably the T-cell compartment to additional viral antigens, such as the nucleocapsid protein, that could contribute to more effective protection against severe COVID-19 [71]. Furthermore, delaying treatment for a short period, perhaps by 3–6 months, to facilitate 1–3% B-cell repletion, and the development of the most effective booster programme possible may be a justifiable risk and could be offered to the immunosuppressed individual to make an informed choice. This could be facilitated by monitoring B-cell repletion and disease activity using imaging. It remains to be seen whether anti-CD20-depleted, but vaccinated individuals remain at any additional risk of severe COVID-19 compared to the general population, and if so, measures discussed here may be warranted. Optimization studies are therefore urgently required so that they can inform on vaccine boosters for immunosuppressed people.
Acknowledgements
This publication is in part based on research using data from data contributors Roche that has been made available through Vivli, Inc. Vivli has not contributed to or approved, and is not in any way responsible for, the contents of this publication. Data analysis, interpretation, and conclusions made are independent from Roche. We thank Alison Schroeer for assistance with production of figures.
Glossary
Abbreviations
- COVID-19
coronavirus disease 2019
- EBV
Epstein-Barr virus
- MS
multiple sclerosis
- PCR
, polymerase chain reaction
- SARS-CoV-2
severe acute respiratory syndrome corona virus two
Contributor Information
David Baker, The Blizard Institute, Barts and The London School of Medicine & Dentistry, Queen Mary University of London, London, UK.
Amy MacDougall, Medical Statistics, London School of Hygiene and Tropical Medicine, London, UK.
Angray S Kang, The Blizard Institute, Barts and The London School of Medicine & Dentistry, Queen Mary University of London, London, UK; Centre for Oral Immunobiology and Regenerative Medicine, Dental Institute, Barts and the London School of Medicine and Dentistry, Queen Mary University of London, London, UK.
Klaus Schmierer, The Blizard Institute, Barts and The London School of Medicine & Dentistry, Queen Mary University of London, London, UK; Clinical Board Medicine (Neuroscience), The Royal London Hospital, Barts Health NHS Trust, London, UK.
Gavin Giovannoni, The Blizard Institute, Barts and The London School of Medicine & Dentistry, Queen Mary University of London, London, UK; Clinical Board Medicine (Neuroscience), The Royal London Hospital, Barts Health NHS Trust, London, UK.
Ruth Dobson, Clinical Board Medicine (Neuroscience), The Royal London Hospital, Barts Health NHS Trust, London, UK; Preventive Neurology Unit, Wolfson Institute of Population Health, Queen Mary University of London, Barts and The London School of Medicine & Dentistry, London, UK.
Funding
This research received no specific funding.
Conflict of Interest
D.B., K.S., G.G., and R.D. have received compensation for consultancy/educational activity from Novartis, or Roche/Genentech who manufacture COVID-19 and MS drugs discussed in this study. These were not involved in the content or the decision to publish. However, Roche received the manuscript to review prior to submission, consistent with the legal agreement required to access the Roche trial data via the Vivli Inc. platform. A.M., A.S.K. have nothing relevant to declare. Although considered irrelevant D.B., K.S., G.G., and R.D. have received compensation for consultancy/educational activity from all companies manufacturing licensed disease-modifying agents in the MS space.
Author contributions
Concept: D.B., A.S.K., K.S., G.G., and R.D.; data extraction: D.B. and A.M.; statistical analysis: A.M.; figure design: D.B. and ASK; initial draft: D.B., A.S.K., and R.B.; manuscript: D.B., A.M., A.S.K., K.S., G.G., and R.D.
Human studies ethics
Clinical trial data (NCT00676715) were collected with ethical approval with informed consent [6, 125].
Data availability
Clinical trial data (NCT00676715/WA21493) are available from Roche/Genentech under contract via the www.vivli.org clinical research data sharing platform.
References
- 1. Cragg MS, Walshe CA, Ivanov AO, Glennie MJ. The biology of CD20 and its potential as a target for mAb therapy. Curr Dir Autoimmun 2005, 8, 140–74. [DOI] [PubMed] [Google Scholar]
- 2. Du FH, Mills EA, Mao-Draayer Y. Next-generation anti-CD20 monoclonal antibodies in autoimmune disease treatment. Auto Immun Highlights 2017, 8, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. VanDerMeid KR, Elliott MR, Baran AM, Barr PM, Chu CC, Zent CS. Cellular cytotoxicity of next-generation CD20 monoclonal antibodies. Cancer Immunol Res 2018, 6, 1150–60. [DOI] [PubMed] [Google Scholar]
- 4. Baker D, Marta M, Pryce G, Giovannoni G, Schmierer K. Memory B cells are major targets for effective immunotherapy in relapsing multiple sclerosis. EBioMedicine 2017, 16, 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baker D, Pryce G, Amor S, Giovannoni G, Schmierer K. Learning from other autoimmunities to understand targeting of B cells to control multiple sclerosis. Brain 2018, 141, 2834–47. [DOI] [PubMed] [Google Scholar]
- 6. Baker D, Pryce G, James LK, Marta M, Schmierer K. The ocrelizumab phase II extension trial suggests the potential to improve the risk: benefit balance in multiple sclerosis. Mult Scler Relat Disord 2020, 44, 102279. [DOI] [PubMed] [Google Scholar]
- 7. Jelcic I, Al Nimer F, Wang J, et al. Memory B cells activate brain-homing, autoreactive CD4+ T cells in multiple sclerosis. Cell 2018, 175, 85–100.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sabatino JJ, Zamvil SS, Hauser SL. B-cell therapies in multiple sclerosis. Cold Spring Harb Perspect Med 2019, 9, a032037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fajnzylber J, Regan J, Coxen K, et al.; Massachusetts Consortium for Pathogen Readiness. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun 2020, 11, 5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Booth A, Reed AB, Ponzo S, et al. Population risk factors for severe disease and mortality in COVID-19: a global systematic review and meta-analysis. PLoS One 2021, 16, e0247461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sormani MP; Italian Study Group on COVID-19 Infection in Multiple Sclerosis . An Italian programme for COVID-19 infection in multiple sclerosis. Lancet Neurol 2020, 19, 481–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Louapre C, Collongues N, Stankoff B, et al.; Covisep Investigators. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol 2020, 77, 1079–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Simpson-Yap S, De Brouwer E, Kalincik T, et al. Associations of disease-modifying therapies with COVID-19 severity in multiple sclerosis. Neurology 2021, 97, e1870–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reder AT, Centonze D, Naylor ML, et al. COVID-19 in patients with multiple sclerosis: associations with disease-modifying therapies. CNS Drugs 2021, 35, 317–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bsteh G, Assar H, Hegen H, et al.; AUT-MuSC Investigators. COVID-19 severity and mortality in multiple sclerosis are not associated with immunotherapy: insights from a nation-wide Austrian registry. PLoS One 2021, 16, e0255316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Langer-Gould A, Smith JB, Li BH; KPSC MS Specialist Group . Multiple sclerosis, rituximab, and COVID-19. Ann Clin Transl Neurol 2021, 8, 938–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salter A, Fox RJ, Newsome SD, et al. Outcomes and risk factors associated with SARS-CoV-2 infection in a North American Registry of patients with multiple sclerosis. JAMA Neurol 2021, 78, 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spelman T, Forsberg L, McKay K, Glaser A, Hillert J. Increased rate of hospitalisation for COVID-19 among rituximab-treated multiple sclerosis patients: a study of the Swedish multiple sclerosis registry. Mult Scler 2021. doi: 10.1177/13524585211026272 [DOI] [PubMed] [Google Scholar]
- 19. Sormani MP, Salvetti M, Labauge P, et al.; Musc-19; Covisep Study Groups. DMTs and Covid-19 severity in MS: a pooled analysis from Italy and France. Ann Clin Transl Neurol 2021a, 8, 1738–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hughes R, Whitley L, Fitovski K, et al. COVID-19 in ocrelizumab-treated people with multiple sclerosis. Mult Scler Relat Disord 2021, 49, 102725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pedotti R, Muros-Le Rouzic E, Raposo C, Schippling S, Jessop N. Understanding the impacts of COVID-19 pandemic in people with multiple sclerosis treated with ocrelizumab. Mult Scler Relat Disord 2021, 55, 103203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gadani SP, Reyes-Mantilla M, Jank L, et al. Discordant humoral and T cell immune responses to SARS-CoV-2 vaccination in people with multiple sclerosis on anti-CD20 therapy. EBioMedicine 2021, 73, 103636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baker D, Roberts CAK, Pryce G, et al. COVID-19 vaccine-readiness for anti-CD20-depleting therapy in autoimmune diseases. Clin Exp Immunol 2020, 202, 149–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tallantyre EC, Vickaryous N, Anderson V, et al. COVID-19 vaccine response in people with multiple sclerosis. Ann Neurol. 2021, 91, 89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baker D, Amor S, Kang AS, Schmierer K, Giovannoni G. The underpinning biology relating to multiple sclerosis disease modifying treatments during the COVID-19 pandemic. Mult Scler Relat Disord 2020, 43, 102174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Majdoubi A, Michalski C, O’Connell SE, et al. A majority of uninfected adults show preexisting antibody reactivity against SARS-CoV-2. JCI Insight 2021, 6, e146316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sharwani K, Sharma R, Krishnan M, et al. Detection of serum cross-reactive antibodies and memory response to SARS-CoV-2 in pre-pandemic and post-COVID-19 convalescent samples. J Infect Dis 2021, 224, 1305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gouma S, Weirick ME, Bolton MJ, et al. Sero-monitoring of health care workers reveals complex relationships between common coronavirus antibodies and SARS-CoV-2 severity. JCI Insight 2021, 6, e150449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jones BE, Brown-Augsburger PL, Corbett KS, et al. The neutralizing antibody, LY-CoV555, protects against SARS-CoV-2 infection in nonhuman primates. Sci Transl Med 2021, 13, eabf1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peter AS, Roth E, Schulz SR, et al. A pair of non-competing neutralizing human monoclonal antibodies protecting from disease in a SARS-CoV-2 infection model. Eur J Immunol 2021. doi: 10.1002/eji.202149374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Corti D, Purcell LA, Snell G, Veesler D. Tackling COVID-19 with neutralizing monoclonal antibodies. Cell 2021, 184, 3086–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. O’Brien MP, Forleo-Neto E, Musser BJ, et al.; Covid-19 Phase 3 Prevention Trial Team. Subcutaneous REGEN-COV antibody combination to prevent Covid-19. N Engl J Med 2021, 385, 1184–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dougan M, Nirula A, Azizad M, et al. ; BLAZE-1 Investigators . Bamlanivimab plus etesevimab in mild or moderate Covid-19. N Engl J Med 2021, 385, 1382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kremer AE, Kremer AN, Willam C, et al. Successful treatment of COVID-19 infection with convalescent plasma in B-cell-depleted patients may promote cellular immunity. Eur J Immunol 2021, 51, 2478–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021, 27, 1205–11. [DOI] [PubMed] [Google Scholar]
- 36. Feng S, Phillips DJ, White T, et al.; Oxford COVID Vaccine Trial Group. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med 2021, 27, 2032–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Molodtsov I, Kegeles E, Mitin A, et al. SARS-CoV-2 specific T cells and antibodies in COVID-19 protection: a prospective study. MedRxiv. doi: 10.1101/2021.08.19.21262278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Israelow B, Mao T, Klein J, et al. Adaptive immune determinants of viral clearance and protection in mouse models of SARS-CoV-2. Sci Immunol 2021, 6, eabl4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vijenthira A, Gong I, Betschel SD, Cheung M, Hicks LK. Vaccine response following anti-CD20 therapy: a systematic review and meta-analysis of 905 patients. Blood Adv 2021, 5, 2624–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ollila TA, Lu S, Masel R, et al. Antibody response to COVID-19 vaccination in adults with hematologic malignant disease. JAMA Oncol 2021, 7, 1714–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zabalza A, Cárdenas-Robledo S, Tagliani P, et al. COVID-19 in multiple sclerosis patients: susceptibility, severity risk factors and serological response. Eur J Neurol 2021, 28, 3384–95. [DOI] [PubMed] [Google Scholar]
- 42. Bigaut K, Kremer L, Fleury M, Lanotte L, Collongues N, de Seze J. Impact of disease-modifying treatments on humoral response after COVID-19 vaccination: a mirror of the response after SARS-CoV-2 infection. Rev Neurol 2021. doi: 10.1016/j.neurol.2021.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van Kempen ZLE, Strijbis EMM, Al MMCT, et al. SARS-CoV-2 antibodies in adult patients with multiple sclerosis in the Amsterdam MS cohort. JAMA Neurol 2021, 78, 880–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Louapre C, Ibrahim M, Maillart E, et al.; COVISEP and Bio-coco-neuroscience study group. Anti-CD20 therapies decrease humoral immune response to SARS-CoV-2 in patients with multiple sclerosis or neuromyelitis optica spectrum disorders. J Neurol Neurosurg Psychiatry 2022, 93, 24–31. [DOI] [PubMed] [Google Scholar]
- 45. Sormani MP, Schiavetti I, Landi D, et al. SARS-CoV-2 serology after COVID-19 in multiple sclerosis: an international cohort study. Mult Scler 2021. doi: 10.1177/13524585211035318 [DOI] [PubMed] [Google Scholar]
- 46. Klineova S, Harel A, Straus Farber R, et al. Outcomes of COVID-19 infection in multiple sclerosis and related conditions: one-year pandemic experience of the multicenter New York COVID-19 Neuroimmunology Consortium (NYCNIC). Mult Scler Relat Disord 2021, 55, 103153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Garcia P, Anand S, Han J, et al. COVID19 vaccine type and humoral immune response in patients receiving dialysis. J Am Soc Nephrol 2022, 33, 33–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ramesh S, Govindarajulu M, Parise RS, et al. Emerging SARS-CoV-2 variants: a review of its mutations, its implications and vaccine efficacy. Vaccines 2021, 9, 1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Achiron A, Mandel M, Dreyer-Alster S, et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther Adv Neurol Disord 2021, 14, 17562864211012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Apostolidis SA, Kakara M, Painter MM, et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat Med 2021, 27, 1990–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gallo A, Capuano R, Donnarumma G, et al. Preliminary evidence of blunted humoral response to SARS-CoV-2 mRNA vaccine in multiple sclerosis patients treated with ocrelizumab. Neurol Sci 2021, 42, 3523–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sormani MP, Inglese M, Schiavetti I, et al.; CovaXiMS Study Group on behalf of the Italian Covid-19 Alliance in MS. Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies. EBioMedicine 2021, 72, 103581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schietzel S, Anderegg MA, Limacher A, et al. Humoral and cellular immune responses upon SARS-CoV-2 vaccines in patients with anti-CD20 therapies: a systematic review and meta-analysis of 1342 patients. MedRxiv 2021. doi: 10.1101/2021.09.30.21264335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. König M, Lorentzen ÅR, Torgauten HM, et al. Humoral immunity to SARS-CoV-2 mRNA vaccination in multiple sclerosis: the relevance of time since last rituximab infusion and first experience from sporadic revaccinations. J Neurol Neurosurg Psychiatry 2021. doi: 10.1136/jnnp-2021-327612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Moor MB, Suter-Riniker F, Horn MP, et al. Humoral and cellular responses to mRNA vaccines against SARS-CoV2 in patients with a history of CD20-B-cell depleting therapy. Lancet Rheumatol 2021, 3, e789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mrak D, Tobudic S, Koblischke M, et al. SARS-CoV-2 vaccination in rituximab-treated patients: B cells promote humoral immune responses in the presence of T-cell-mediated immunity. Ann Rheum Dis 2021, 80, 1345–50. [DOI] [PubMed] [Google Scholar]
- 57. Högelin AK, Ruffin N, Elisa P, et al. Humoral and cellular immunological memory against SARS-CoV-2 despite B-cell depleting treatment in multiple sclerosis. iScience 2021, 24, 103078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Monin L, Laing AG, Muñoz-Ruiz M, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol 2021, 22, 765–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Madelon N, Lauper K, Breville G, et al. Robust T cell responses in anti-CD20 treated patients following COVID-19 vaccination: a prospective cohort study. Clin Infect Dis 2021. doi: 10.1093/cid/ciab954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Disanto G, Sacco R, Bernasconi E, et al. Association of disease-modifying treatment and anti-CD20 infusion timing with humoral response to 2 SARS-CoV-2 vaccines in patients with multiple sclerosis. JAMA Neurol 2021, 78, 1529–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kornek B, Leutmezer F, Rommer PS, et al. Distinct patterns of humoral and cellular immune responses following SARS-CoV-2 mRNA vaccination in patients with immune-mediated neurological disorders on anti-CD20 therapy: a prospective cohort study. SSRN 2021. doi: 10.2139/ssrn.3924204 [DOI] [Google Scholar]
- 62. Stefanski AL, Rincon-Arevalo H, Schrezenmeier E, et al. B cell numbers predict humoral and cellular response upon SARS-CoV-2 vaccination among patients treated with rituximab. MedRxiv. doi: 10.1101/2021.07.19.21260803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Henriquez S, Zerbit J, Bruel T, et al. Anti-CD38 therapy impairs SARS-CoV-2 vaccine response in multiple myeloma patients. MedRxiv. doi: 10.1101/2021.08.08.21261769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ghandili S, Schönlein M, Lütgehetmann M, et al. Post-vaccination anti-SARS-CoV-2-antibody response in patients with multiple myeloma correlates with low CD19+ B-lymphocyte count and anti-CD38 treatment. Cancers 2021, 13, 3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Iannetta M, Landi D, Cola G, et al. T-cell responses to SARS-CoV-2 in multiple sclerosis patients treated with ocrelizumab healed from COVID-19 with absent or low anti-spike antibody titers. Mult Scler Relat Disord 2021, 55, 103157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schwarz T, Otto C, Jones TC, et al. A cohort of 222 anti-CD20 treated patients with multiple sclerosis followed through the COVID-19 pandemic: attenuated humoral but robust cellular immune responses after vaccination and infection. MedRXiv 2021. doi: 10.1101/2021.10.11.21264694 [DOI] [Google Scholar]
- 67. Brill L, Rechtman A, Zveik O, et al. Humoral and T-cell response to SARS-CoV-2 vaccination in patients with multiple sclerosis treated with ocrelizumab. JAMA Neurol 2021, 78, 1510–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bange EM, Han NA, Wileyto P, et al. CD8+ T cells contribute to survival in patients with COVID-19 and hematologic cancer. Nat Med 2021, 27, 1280–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Oberhardt V, Luxenburger H, Kemming J, et al. Rapid and stable mobilization of CD8+ T cells by SARS-CoV-2 mRNA vaccine. Nature 2021, 597, 268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Le Bert N, Tan AT, Kunasegaran K, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020, 584, 457–62. [DOI] [PubMed] [Google Scholar]
- 71. Grifoni A, Sidney J, Vita R, et al. SARS-CoV-2 human T cell epitopes: adaptive immune response against COVID-19. Cell Host Microbe 2021, 29, 1076–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wei J, Stoesser N, Matthews PC, et al.; COVID-19 Infection Survey Team. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom. Nat Microbiol 2021, 6, 1140–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Shrotri M, Navaratnam AMD, Nguyen V, et al.; Virus Watch Collaborative. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet 2021, 398, 385–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bergwerk M, Gonen T, Lustig Y, et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med 2021, 385, 1474–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Goldberg Y, Mandel M, Bar-On YM, et al. Waning immunity of the BNT162b2 vaccine: a nationwide study from Israel. N Engl J Med 2021, 385, e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cook C, Patel N, D’Silva K, et al. Clinical characteristics and outcomes of COVID-19 breakthrough infections among vaccinated patients with systemic autoimmune rheumatic diseases. Ann Rheum Dis 2021, 81, 289–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Di Fusco M, Moran MM, Cane A, et al. Evaluation of COVID-19 vaccine breakthrough infections among immunocompromised patients fully vaccinated with BNT162b2. MedRxiv 2021. doi: 10.1101/2021.10.12.21264707 [DOI] [PubMed] [Google Scholar]
- 78. Kearns P, Siebert S, Willicombe M, et al. Examining the immunological effects of COVID-19 vaccination in patients with conditions potentially leading to diminished immune response capacity—the OCTAVE trial. SSRN 2021. doi: 10.2139/ssrn.3910058 [DOI] [Google Scholar]
- 79. Shroff RT, Chalasani P, Wei R, et al. Immune responses to COVID-19 mRNA vaccines in patients with solid tumors on active, immunosuppressive cancer therapy. MedRxiv 2021. doi: 10.1101/2021.05.13.21257129 [DOI] [Google Scholar]
- 80. Re D, Seitz-Polski B, Carles M, et al. Humoral and cellular responses after a third dose of BNT162b2 vaccine in patients treated for lymphoid malignancies. MedRxiv 2021. doi: 10.1101/2021.07.18.21260669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Connolly CM, Teles M, Frey S, et al. Booster-dose SARS-CoV-2 vaccination in patients with autoimmune disease: a case series. Ann Rheum Dis 2021, 81, 291–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Greenberger LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL. Anti-spike antibody response to SARS-CoV-2 booster vaccination in patients with B cell-derived hematologic malignancies. Cancer Cell 2021, 39, 1297–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. König M, Torgauten HM, Tran TT, et al. Immunogenicity and safety of a third SARS-CoV-2 vaccine dose in patients with multiple sclerosis and weak immune response after COVID-19 vaccination. JAMA Neurol 2022. doi: 10.1001/jamaneurol.2021.5109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Spiera R, Jinich S, Jannat-Khah D. Rituximab, but not other antirheumatic therapies, is associated with impaired serological response to SARS- CoV-2 vaccination in patients with rheumatic diseases. Ann Rheum Dis 2021, 80, 1357–9. [DOI] [PubMed] [Google Scholar]
- 85. Palanichamy A, Jahn S, Nickles D, et al. Rituximab efficiently depletes increased CD20-expressing T cells in multiple sclerosis patients. J Immunol 2014, 193, 580–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Leandro MJ, Cambridge G, Ehrenstein MR, Edwards JC. Reconstitution of peripheral blood B cells after depletion with rituximab in patients with rheumatoid arthritis. Arthritis Rheum 2006, 54, 613–20. [DOI] [PubMed] [Google Scholar]
- 87. Bar-Or A, Wiendl H, Montalban X, et al. Rapid and sustained B-cell depletion with subcutaneous ofatumumab in relapsing multiple sclerosis: APLIOS, a randomized phase-2 study. Mult Scler 2021. doi: 10.1177/13524585211044479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kesimpta®. Summary of Medical Product Characteristics/Label. European Medicines Agency. 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125326s070lbl.pdf (24 October 2021, date last accessed). [Google Scholar]
- 89. Gibiansky E, Petry C, Mercier F, et al. Ocrelizumab in relapsing and primary progressive multiple sclerosis: pharmacokinetic and pharmacodynamic analyses of OPERA I, OPERA II and ORATORIO. Br J Clin Pharmacol 2021, 87, 2511–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Genovese MC, Kaine JL, Lowenstein MB, et al.; ACTION Study Group. Ocrelizumab, a humanized anti-CD20 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: a phase I/II randomized, blinded, placebo-controlled, dose-ranging study. Arthritis Rheum 2008, 58, 2652–61. [DOI] [PubMed] [Google Scholar]
- 91. Ellwardt L, Bittner S, Zipp F. Monitoring B-cell repopulation after depletion therapy in neurologic patients. Neurol Neuroimmunol Neuroinflamm 2018; 5, e463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ellrichmann G, Bolz J, Peschke M, et al. Peripheral CD19+ B-cell counts and infusion intervals as a surrogate for long-term B-cell depleting therapy in multiple sclerosis and neuromyelitis optica/neuromyelitis optica spectrum disorders. J Neurol 2019, 266, 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Signoriello E, Bonavita S, Di Pietro A, et al. BMI influences CD20 kinetics in multiple sclerosis patients treated with ocrelizumab. Mult Scler Relat Disord 2020, 43, 102186. [DOI] [PubMed] [Google Scholar]
- 94. Nakou M, Katsikas G, Sidiropoulos P, et al. Rituximab therapy reduces activated B cells in both the peripheral blood and bone marrow of patients with rheumatoid arthritis: depletion of memory B cells correlates with clinical response. Arthritis Res Ther 2009, 11, R131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bar-Or A, Calabresi PA, Arnold D, et al. Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol 2008, 63, 395–400. [DOI] [PubMed] [Google Scholar]
- 96. Sahi NK, Abidi SMA, Salim O, Abraham R, Kalra S, Al-Araji A. Clinical impact of ocrelizumab extended interval dosing during the COVID-19 pandemic and associations with CD19+B-cell repopulation. Mult Scler Relat Disord 2021, 56, 103287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Maarouf A, Rico A, Boutiere C, et al.; Under the Aegis of OFSEP. Extending rituximab dosing intervals in patients with MS during the COVID-19 pandemic and beyond? Neurol Neuroimmunol Neuroinflamm 2020, 7, e825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Rolfes L, Pawlitzki M, Pfeuffer S, et al. Ocrelizumab extended interval dosing in multiple sclerosis in times of COVID-19. Neurol Neuroimmunol Neuroinflamm 2021, 8, 1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Tazza F, Lapucci C, Cellerino M, et al. Personalizing ocrelizumab treatment in multiple sclerosis: what can we learn from SARS-CoV-2 pandemic? J Neurol Sci 2021, 427, 117501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Chertcoff A, Bauer J, Silva BA, et al. Changes on the health care of people with multiple sclerosis from Latin America during the COVID-19 pandemic. Mult Scler Relat Disord 2021, 54, 103120. [DOI] [PubMed] [Google Scholar]
- 101. van Lierop ZY, Toorop AA, van Ballegoij WJ, et al. Personalized B-cell tailored dosing of ocrelizumab in patients with multiple sclerosis during the COVID-19 pandemic. Mult Scler 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Barun B, Gabelić T, Adamec I, et al. Influence of delaying ocrelizumab dosing in multiple sclerosis due to COVID-19 pandemics on clinical and laboratory effectiveness. Mult Scler Relat Disord 2021, 48, 102704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Schulz E, Hodl I, Forstner P, et al. Association of naive B cells with humoral response to SARS-CoV-2 vaccination. MedRxiv. doi: 10.1101/2021.08.11.21261898 [DOI] [Google Scholar]
- 104. Gurion R, Rozovski U, Itchaki G, et al. Humoral serologic response to the BNT162b2 vaccine is abrogated in lymphoma patients within the first 12 months following treatment with anti-CD2O antibodies. Haematologica 2021. doi: 10.3324/haematol.2021.279216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Famulare M. Seroconversion after COVID-19 vaccination in patients using B-cell depleting therapies to manage multiple sclerosis increases with time between treatment and vaccination. Github v0.2 2021, https://github.com/famulare/covid-vax-response-vs_time_since_bcdt. [Google Scholar]
- 106. Baker D, MacDougall A, Kang AS, Schmierer K, Giovannoni G, Dobson R. CD19 B cell repopulation after ocrelizumab, alemtuzumab and cladribine: implications for SARS-CoV-2 vaccinations in multiple sclerosis. MedRxiv 2021. doi: 10.1101/2021.09.26.21264023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kletzl H, Gibiansky E, Petry C, et al. Pharmacokinetics, pharmacodynamics and exposure-response analyses of ocrelizumab in patients with multiple sclerosis (N4.001). Neurology 2019, 92, N4.001. [Google Scholar]
- 108. Kim SH, Hyun JW, Kim HJ. Individualized B cell-targeting therapy for neuromyelitis optica spectrum disorder. Neurochem Int 2019, 130, 104347. [DOI] [PubMed] [Google Scholar]
- 109. Novi G, Bovis F, Fabbri S, et al. Tailoring B cell depletion therapy in MS according to memory B cell monitoring. Neurol Neuroimmunol Neuroinflamm 2020, 7, e845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Yavuz S, Komsuoğlu Çelikyurt FI. Antiviral treatment of COVID-19: an update. Turk J Med Sci 2021, 51, 3372–90. doi: 10.3906/sag-2106-250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Mahase E. Covid-19: molnupiravir reduces risk of hospital admission or death by 50% in patients at risk, MSD reports. BMJ 2021, 375, n2422. [DOI] [PubMed] [Google Scholar]
- 112. Hurt AC, Wheatley AK. Neutralizing antibody therapeutics for COVID-19. Viruses 2021, 13, 628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Drouin AC, Theberge MW, Liu SY, et al. Successful clearance of 300 day SARS-CoV-2 infection in a subject with B-cell depletion associated prolonged (B-DEAP) COVID by REGEN-COV anti-spike monoclonal antibody cocktail. Viruses 2021, 13, 1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Dong J, Zost SJ, Greaney AJ, et al. Genetic and structural basis for SARS-CoV-2 variant neutralization by a two-antibody cocktail. Nat Microbiol 2021, 6, 1233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Astrazeneca.com. 2021. https://www.astrazeneca.com/media-centre/press-releases/2021/update-on-azd7442-storm-chaser-trial.html (29 January 2022, date last accessed).
- 116. Luitel P, Vais D, Gidron A. Successful treatment of persistent coronavirus disease 2019 infection in a patient with hypogammaglobulinemia with REGN-COV2: a case report. Open Forum Infect Dis 2021, 8, ofab335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Lazarevic I, Pravica V, Miljanovic D, Cupic M. Immune evasion of SARS-CoV-2 emerging variants: what have we learnt so far? Viruses 2021, 13, 1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Planas D, Veyer D, Baidaliuk A, et al. Reduced sensitivity of SARS-CoV-2 variant delta to antibody neutralization. Nature 2021, 596, 276–80. [DOI] [PubMed] [Google Scholar]
- 119. Avanzato VA, Matson MJ, Seifert SN, et al. Case study: prolonged infectious SARS-CoV-2 shedding from an asymptomatic immunocompromised individual with cancer. Cell 2020, 183, 1901–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Pan D, Mudalige NL, Sze S, et al. The new UK SARS-CoV-2 variant and lockdown – causes and consequences. Clin Med 2021, 21, e295–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Corey L, Beyrer C, Cohen MS, Michael NL, Bedford T, Rolland M. SARS-CoV-2 variants in patients with immunosuppression. N Engl J Med 2021, 385, 562–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Gibson EG, Pender M, Angerbauer M, et al. Prolonged SARS-CoV-2 illness in a patient receiving ocrelizumab for multiple sclerosis. Open Forum Infect Dis 2021, 8, ofab176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Kemp SA, Collier DA, Datir RP, et al.; CITIID-NIHR BioResource COVID-19 Collaboration; COVID-19 Genomics UK (COG-UK) Consortium. SARS-CoV-2 evolution during treatment of chronic infection. Nature 2021, 592, 277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Giovannoni G, Hawkes CH, Lechner-Scott J, Levy M, Yeh EA, Baker D. COVID-19 vaccines and multiple sclerosis disease-modifying therapies. Mult Scler Relat Disord 2021, 53, 103155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kappos L, Li D, Calabresi PA, et al. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet 2011, 378, 1779–87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Clinical trial data (NCT00676715/WA21493) are available from Roche/Genentech under contract via the www.vivli.org clinical research data sharing platform.