Abstract
The lymphatic system plays an integral part in regulating immune cell trafficking and the transport of macromolecules. However, its influence on disease progression and drug uptake is understood less than that of the vascular system. To bridge this knowledge gap, biomaterials can be used to investigate the lymphatic system and to provide novel understanding into complex disease processes, including cancer metastasis and inflammation. Insight gained from these mechanistic studies can be further used to design innovative biomaterials to modulate the immune system, improve drug delivery, and promote tissue regeneration. This review article focuses on recent advances in (i) biomaterials used for lymphatic vessel formation, (ii) models for studying lymphatic-immune cells interactions, (iii) pharmaceuticals and their interactions with the lymphatic system, (iv) and strategies for drug delivery via the lymphatic system. Finally, several challenges regarding adopting biomaterials for immunomodulation and future perspectives are discussed.
Keywords: Biomaterials, Lymphatic Vessels, Tissue Engineering, Immune System, Drug Delivery
Graphic Abstract
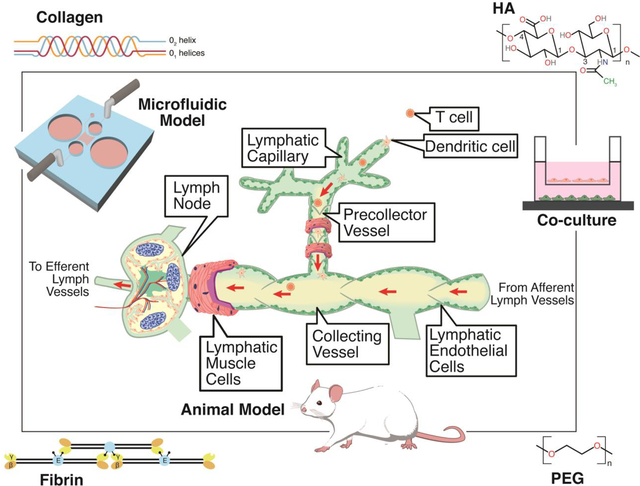
1. Overview of the Lymphatic System
The lymphatic system is an integral part of the circulatory system which is comprised of a network of lymphatic vasculature and lymphoid organs. This system is responsible for fluid homeostasis and the transport and exchange of an array of cells and molecules, including immune cells, nutrients, and long-chain fatty acids [1]. Dendritic cells (DCs), T cells, and antigens enter the system via the lymphatic capillaries due in part to forces from the interstitial fluid (IF). These small, blind-ended vessels are composed of a single layer of lymphatic endothelial cells (LECs), which are leaf-like in structure, and possess discontinuous button-like cell-cell junctions [2–4]. These gaps allow for the nonselective uptake of tissue fluid into the lymphatic microvasculature [2,3]. Moreover, the interaction between Lymphatic Vessel Endothelial Receptor-1 (LYVE-1) within these loose junctions and hyaluronan/hyaluronic acid (HA) glycocalyx on leukocytes causes modulation of the lymphatic vessels which allows leukocytes to enter [5]. Pressure caused by fluid moving through the interstitium and systemic factors, including arterial and skeletal muscle contractions, helps to drive the formation of lymph [6]. DCs were found to actively crawl through the lymphatic capillaries, sometimes even in the opposite direction of the lymph flow, until they detached from the wall and returned to a passive form of transport [4]. Since both DCs and neutrophils were found to follow this active form of transport, it is likely that the T cells also crawl within the vessels [3].
The lymph and cells are then driven by a pressure gradient caused by IF into the pre-collecting and collecting vessels. Unlike lymphatic capillaries, these collecting vessels have continuous zipper-like cell-cell junctions, intraluminal valves that prevent the backflow of lymph, and are surrounded by a contractile layer of non-striated muscle cells, referred to as lymphatic muscle cells (Figure 1). Even though only the button-like cell-cell junctions allow for intravasation, both types of junctions are comprised of vascular endothelial cadherin (VE-cadherin) and proteins associated with tight junctions, including occludin, claudin-5, and endothelial cell–selective adhesion molecule [7]. The lymphatic muscle cells surrounding these vessels contracts to cause a pressure gradient, which drives the lymph, including immune cells, towards the lymph node (LN) and is referred to as the afferent flow.
Figure 1. Multiple types of models can be used to better understand the lymphatic system and its influence on disease progression, immune response, and drug uptake.
(A) These models include co-cultures, microfluidics, and animal models to develop and study lymphatic vessels. Biomaterials, specifically collagen, hyaluronic acid (HA), poly(ethylene glycol) (PEG), and fibrin, are utilized to develop biomimetic environments conducive to lymphatic vessels. Lymphatic vessels are composed of LECs that form lymphatic capillaries, which are characterized by discontinuous, button-like endothelial cell junctions, and pre-collecting and collecting vessels, which exhibit continuous, zipper-like cell-cell junctions and are surrounded by a contractile layer of non-striated muscle cells, referred to as lymphatic muscle cells that propel the flow of lymph to the LNs and contain valves to prevent backflow. Immune cells can enter through the capillaries and flow with lymph into the LNs where they are stored until an immune response triggers them to egress and reenter circulation. (B) A zoomed-in view of the structures within the LN. The parenchyma encompasses all of the internal functional tissue, which is comprised of reticular fibers, and is demarcated with the red outline. The sectional view (i) of the LN focuses on the structures that are utilized for immune cell entry, storage, and egress.
The LN subcapsular sinus (SCS) receives lymph from several afferent collecting vessels. Lymphocytes, including both B and T cells, are able to enter the LN through blood carried via high endothelial venules (HEVs) [3,8]. DCs can transmigrate through the SCS floor into the LN parenchyma and cause local changes that allow for the T cells to enter [3,9]. However, T cells tend to enter from the peripheral medullary sinuses. The immune cells then use CCR7 chemokine receptors to migrate to the T cell zone or CXCR5 chemokines to travel to the B cell zone [8–10]. Antigens enter the LN via subcapsular macrophages or a network of small conduits made of fibroblastic reticular cells (FRC) that connect the subcapsular and paracortical sinuses, known as the reticular network [11]. Antigens enter the B cell zone and are internalized by B cells or DCs [10].
When an immune response occurs, immune cells migrate back into the sinuses and either actively crawl or flow with the lymph into the efferent flow, which is flow directed away from the LNs [3,4]. The efferent flow goes through a chain of LNs and eventually the immune cells reenter the bloodstream to be brought to the site of inflammation. While the afferent lymph contains mostly T cells and DCs, the efferent lymph has more B cells, which come from the B cell follicles located in the cortex of the LN [12].
Given the structural complexity and dynamics of the lymphatic system, as well as its involvement with immune functions and disease progression, more physiologically relevant models with the ability to incorporate biomechanical cues, 3-dimensional (3-D) structures, biochemical factors, and additional cell types are needed. Biomaterials present the capability of creating these models, which will be discussed in the following sections.
2. Biomaterial-based tissue engineering approaches for lymphatic vessel engineering
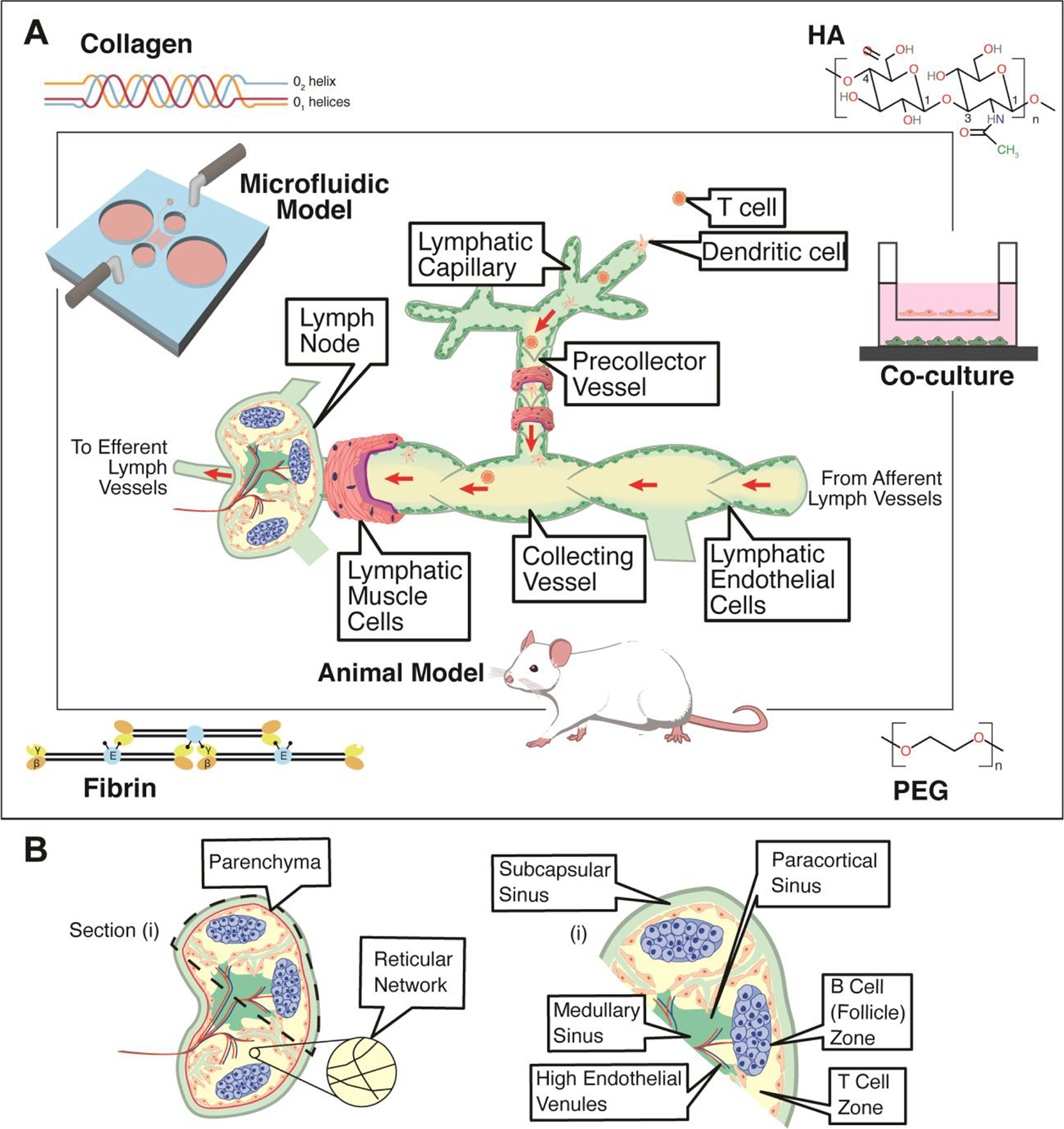
One of the most notable advantages of biomaterials-based models for lymphatic studies is the ability to achieve a range of physiologically relevant stiffnesses and investigate how matrix stiffness influences lymphangiogenesis or disease processes. Frye et al. demonstrated that matrix stiffness regulates VEGFR-3 expression and that soft matrices upregulated pro-lymphangiogenic genes [13], which underscores the need for new models with matrix stiffnesses similar to in vivo conditions. Certain biomaterials also present the ability to decouple stimuli and investigate the contributions of specific factors, or to spatially or temporally incorporate specific factors. By controlling ligand binding sites and mechanical properties [14], hyaluronic acid-based hydrogels (HA-hydrogels) were engineered to generate lymphatic cord-like structures from LECs (Figure 2A–C) and again demonstrated how matrix stiffness is a critical factor for lymphatic studies. The versatility of particular advantages of HA-hydrogels are discussed in the following sections.
Figure 2. Biomaterials to modulate the lymphatic system.
(A) Matrix stiffness of hyaluronic acid (HA)-hydrogels prime lymphatic tube formation directed by VEGF-C, as demonstrated by fluorescent microscopy of F-actin (green) and nuclei (blue). Scale bars are 50 μm. (B) Confocal images of lymphatic tubes formed on soft HA-hydrogels showing junctional markers for CD31 and VE-Cad. Enlarged rendering of confocal image stacks indicate cellular junctions (arrowheads) with discontinuous (arrows) and overlapping (asterisks) junctions. Scale bars are 50 μm and 25 μm (inset). (C) TEM analyses of lymphatic tubes formed after 12 hours showed LECs degrading the HA-hydrogels (H) to generate intracellular vacuoles (V), some of which were observed in the process of merging (asterisk) into coalescent vacuoles (CV). Scale bar is 20 μm. Illustration was adapted with permission from [26]. (D) FACS-sorted LECs mixed with 40% fibroblasts developed lymphatic capillaries (CD31, red) within Collagen type-1. (E) Lymphatic capillaries expressed the lymphatic marker Prox-1 (green). Scale bars are 40 μm. Illustration was adapted with permission from [34].
2.1. Hyaluronan and leukocyte trafficking
HA-hydrogels present great promise, either as a stand-alone therapy or as a scaffold and delivery system for tissue engineering approaches for lymphatic vessels [15]. HA is a non-sulphated glycosaminoglycan that contains repeating disaccharide units of N-acetylglucosamine and glucuronic acid [16] and is found abundantly during embryogenesis where it has a crucial role in regulating angiogenesis, lymphangiogenesis, and organ morphogenesis [17]. HA is ubiquitous in the extracellular matrix (ECM), is versatile and can be chemically modified, and is non-immunogenic, making HA attractive and widely used in tissue engineering and medicine [18–21]. HA-hydrogels present a unique advantage for lymphatic vessel engineering since CD44 binds to HA and LECs highly express LYVE-1 which is a CD44 homologue [14,22]. Due to its developmental relevance, importance for LECs, and ability to support viable cells, HA-hydrogels serve as an excellent substrate to control lymphatic vascular morphogenesis in a biomimetic environment, where they can be potentially developed as transplant options or as advanced in vitro models [23–25]. Furthermore, HA and LYVE-1 play an important role in leukocyte trafficking, which additionally supports the potential of HA-hydrogels for model studies involving immune trafficking and the lymphatic system.
HA is particularly relevant for immune cell trafficking through lymphatic vasculature since CD44 serves as the principle receptor for HA [26], and it has recently been reported that DC trafficking in lymphatics can be dynamically mediated through CD44/HA interactions [27]. Furthermore, LYVE-1 has been identified as a docking receptor for DCs on the lymphatic endothelium. The process of leukocyte trafficking into the lymphatic capillaries is mediated by the LYVE-1/HA axis, where HA on the leukocyte surface binds with LYVE-1 on the lymphatic endothelium, the interdigitating endothelial flaps open during leukocyte entry, and leukocytes then migrate to downstream LNs for immune activation [5,28]. Additionally, T cells and DCs bind to each other via HA, and CD44 can serve as a mediator [29]. Modification of this CD44-HA interaction also poses as an anti-inflammatory treatment strategy through the blockade of neutrophil recruitment [28].
2.2. Biomaterials in lymphatic vessel tissue engineering approaches
Beyond the advantages of HA for lymphatic tissue engineering, the versatility and possibility of numerous chemical modifications make HA an attractive platform. One particularly promising approach is to modify HA with norbornene groups which allows for both mechanical and biochemical modulation, as well as sequential spatial patterning of peptides, and can be used to develop a more complex in vitro system [30]. In a myocardial infarction (MI) mouse model, injection of HA-hydrogels post-MI resulted in decreased scarring and collagen deposition, demonstrating the potential in vivo applications [31].
In addition to HA-hydrogels, multiple other biomaterial options have been employed in tissue engineering approaches to generate lymphatic vasculature in vitro or in vivo, including collagen, fibrin, alginate, and poly(ethylene glycol) diacrylate (PEGDA). Fibrin and collagen have commonly been used in combinatorial approaches to generate lymphatic capillaries vessels in vitro. With the addition of interstitial flow for ten days, lymphatic vessels developed in both fibrin and collagen but showed preference towards fibrin hydrogels [32]. In another fibrin-collagen model, premature lymphatic vessels were observed in vitro after 21 days (Figure 2D–E) [33]. Beyond using collagen and fibrin to develop lymphatic vessels, immune models can be created. Collagen scaffolds with DCs and thymic stromal cells were transplanted into mice and formed organoids representative of secondary lymphoid tissues. These organoids possessed T and B cell zones, HEV-like vessels, and DC networks that were able to mount humoral and cellular immune responses, in addition to antigen specific secondary antibody responses [34,35].
Progressing to in vivo applications, BioBridge, an aligned nanofibrillar collagen scaffold, increased the density of lymphatic collecting vessels in a porcine model whereas the control subjects developed fibrous scar tissue [36]. Despite lymphatic vessel density increasing with the BioBridge and either VEGF-C supplementation or lymph node transplantation, VEGF-C supplementation decreased functionality, as measured by bioimpedance. This paradoxical result highlights the complexity of VEGF-C delivery which requires spatial and temporal regulation. Biomaterials can provide both types of regulation and could be used to address this challenge.
Another natural polymer, alginate, has been used in applications to both promote and inhibit lymphangiogenesis. Used as an injectable hydrogel for the controlled release of VEGF-C, lymphangiogenesis was induced in a chick chorioallontoic membrane assay [37]. In another model, anti-VEGFR-3 siRNA was delivered via polyethylenimine-alginate nanoparticles and demonstrated inhibition of lymphatic vessel formation [34].
PEGDA has been widely used in tissue engineering and is mechanically tunable, which provides a platform to investigate substrate mechanics while maintaining the same composition of binding sites. This feature provides an advantage compared to natural biomaterials like collagen, fibrin, and alginate which cannot be mechanically modified without modifying the composition. Specific uses of PEGDA will be discussed in the models section of this review article.
2.3. Biomaterials in drug delivery via the lymphatic system
Beyond hydrogels for tissue-engineered models of lymphatic vasculature, various biomaterials have also been used to design cryogels, matrices that are polymerized at sub-zero temperatures, for vaccines or nanoparticle delivery systems to target immunomodulation through the lymphatic system. Cryogels serve as an injectable biomaterial vaccine option and can be constructed from alginate, gelatin, polyethylene glycol, HA, or other cross-linkable polymers. Antigens can be loaded within these cryogels and used to activate DCs which will then migrate to draining lymph nodes (dLNs) [38]. Presently, cryogel-based vaccines have already demonstrated efficacy in breast cancer, melanoma, and leukemia models and provide a potential strategy for future immunomodulation approaches [39].
In addition to cryogels, fatty acids, waxes, monoglycerides, diglycerides, and triglycerides can be used in nanoparticle delivery systems. Compared to colloidal carrier systems which involve a substance dispersed in a solution, lipid-based nanoparticles or carriers exhibit controlled release properties as well as improved chemical stability, which make lipid-based nanoformulations advantageous for lymphatic delivery. Both the lipid type and emulsifier concentration must be considered in the design for effective delivery via the lymphatic system [40].
3. Lymphatic Models to Study Immune Cell Interaction
Both in vitro and in vivo models can be used to model the lymphatic system and its interaction with the immune system. In this section, three different categories of models will be reviewed, including their advantages and disadvantages, as well as advancements made to date.
3.1. Co-culture
Co-culture systems have been utilized for promoting the formation of lymphatic vessels, modeling trans endothelial flow, and modulating the tolerogenic responses. Several improvements have been made to the co-culture system throughout the years. In its most basic form, co-cultures highlight cell-cell interactions of two or more cell types and provide more insight than a monoculture can. For example, Cohen et al cultured FH T cells, which are tyrosinase tolerogenic T cells, with LECs harvested from LNs and peripheral tissues [41]. Through the co-culture system, they were able to see changes in T cell proliferation rates when cultured with different tissues which highlighted the LN microenvironment’s role in the induction of the tolerogenic properties of LECs derived from LNs (LN-LECs). Khosravi-Maharlooei et al. focused on DCs and their ability to migrate to lymphatic tissues in vivo. This group used a co-culture of fibroblasts with DCs to induce DC migration to lymphatic tissues and present fibroblast antigens. They were able to induce a tolerogenic effect, decrease T cell proliferation, and treat cells for in vivo testing [42].
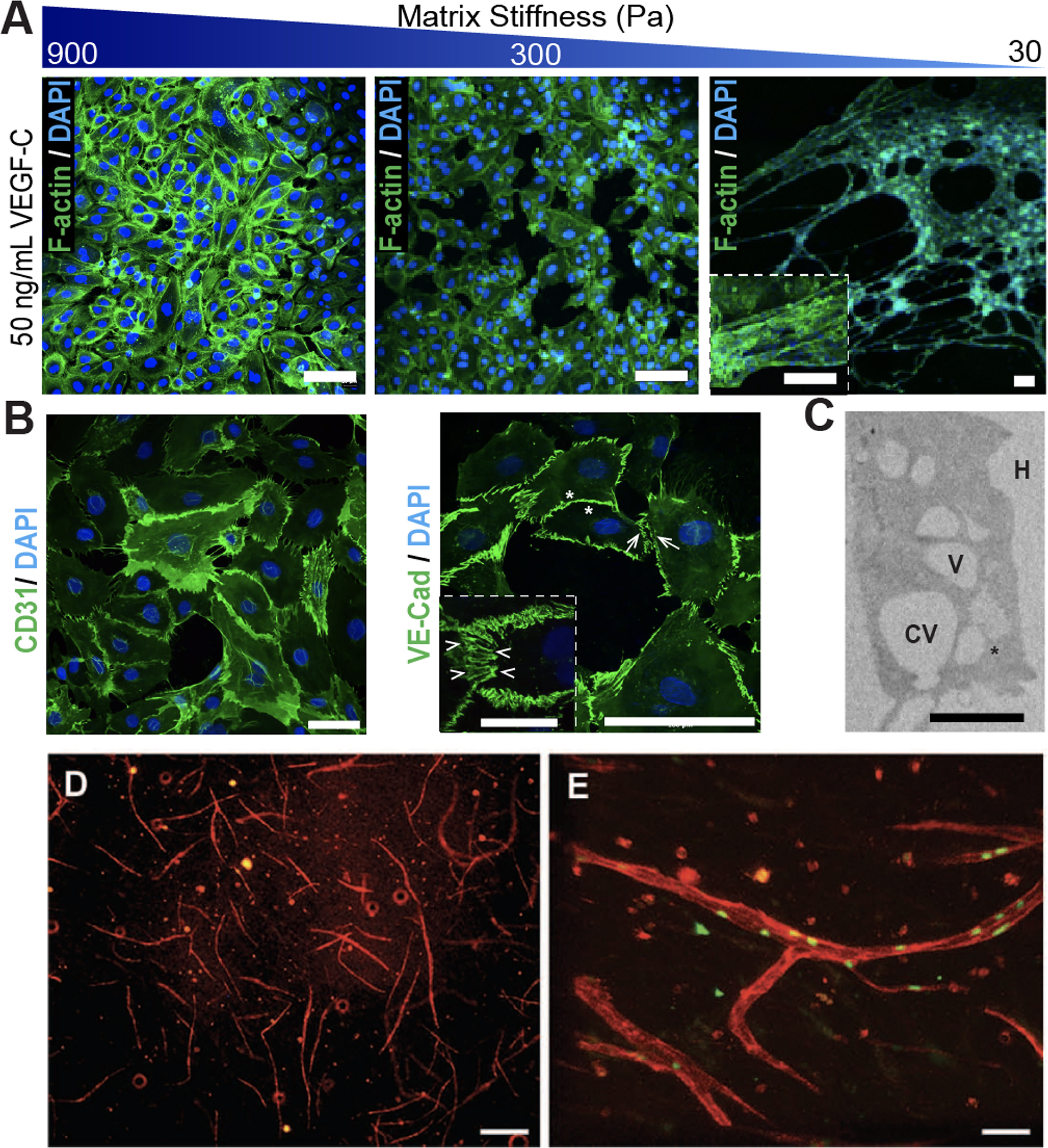
Other researchers have introduced biophysical factors to co-cultures. Shields et al. co-cultured LECs with either breast cancer or melanoma tumor cells and exposed them to simulated interstitial flow to investigate the movement of tumor cells caused by chemokines (Figure 3A–B) [44]. This system was one of the first to show that tumor cells respond to CCR7 ligands and identified the importance of the tumor-ECM-lymphatic microenvironment. This model allowed for better assessment of tumor migration, compared to tumor xenograft models that were previously used, and improved chemotaxis assays by allowing the inclusion of biophysical factors, such as flow and ECM, that can affect cellular transport.
Figure 3. Co-culture and Microfluidic systems to model the lymphatic system.
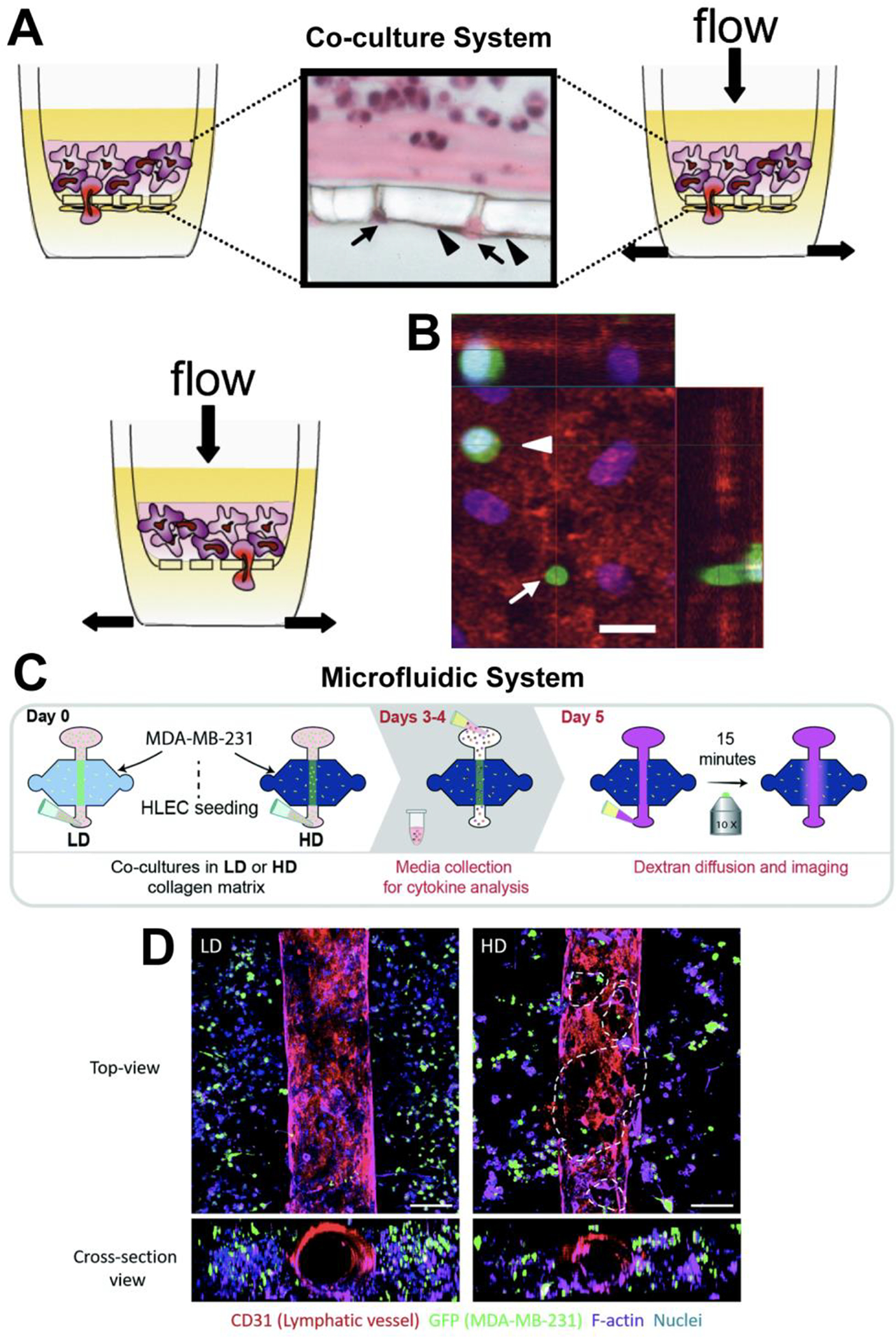
(A) Co-culture system with a 3D matrix and flow to investigate crosstalk between LECs and tumor cells. The cross-section shows the interface for the tumor suspension, porous membrane, and LECs, which are indicated with the black arrowheads. The black arrows indicate tumor cells migrating through the membrane’s pores. (B) Confocal microscopy of the underside of a transwell membrane. The LEC monolayer is stained with CD31 in red and the tumor cells were stained with PHAKT-GFP, a fluorescent protein that binds to AKT protein kinase and selectively migrates to the membrane when exposed to a chemoattractant. One of these tumor cells is adhering (the white arrowhead) and one is transmigrating through a pore (white arrow). The nuclei are stained blue, and the scale bar is 20 μm. Illustration was adapted with permission from [44]. (C) Microfluidics system that contain low-density (LD) or high-density (HD) collagen gels, LECs, and MDA-MB-231 cells, a metastatic breast cancer cell line. (D) Top-view and cross-section view of immunofluorescent images of lymphatic vessels co-cultured with MDA-MB-231 in LD (left) and HD (right) matrices. F-actin was stained with purple, CD31 with red, MDA-MB-231-GFP with green, and the nuclei with blue. The dashed lines indicate where LEC detachment was present in the vessel walls. The scale bar is 140 μm. Illustration was adapted with permission from [65].
Co-cultures can also be used to create vasculature networks. Gibot et al. demonstrated that LECs cultured with fibroblasts were able to form 3-D lymphatic vessel networks, representative of native ones, without the introduction of exogenous factors [45]. Unlike other models, this network did not require extra scaffolds, but instead used multiple layers of fibroblast-LEC co-cultures to create a 3-D network. This model also improved upon previous 2-D cell culture models and 3-D tissue assays which could only develop simple cords or tubes. Co-culture models are a relatively inexpensive, established system for integrating cell-cell interactions into a study. While improvements have been made, there are still several challenges. If both cell lines do not use the same media formulation, at least one cell type will be grown in suboptimal conditions and it may cause changes in proliferation or phenotypes [46]. Another challenge is determining which cell types contribute to phenotypic marker expression or proliferation [47]. Currently, cells are stained with vital dyes before being added to co-cultures, but nanoparticles could be used as a potential alternative. Additionally, it is difficult to determine which signaling effects result from cell-cell contact versus paracrine signaling. Currently, studies that are based on paracrine signaling use physical separation of the cells, but this is not feasible for studies that are dependent on cell-cell signaling [48].
Biomaterials are currently being integrated into co-culture systems. This is done primarily through scaffolds, which help support the structures the cells form. For example, Knezevic et al. used a co-culture of adipose-derived stromal cells (ASCs), LECs, and blood endothelial cells (BECs) on a fibrin-based scaffold to study the integration of blood and lymphatic vasculature. The BECs and LECs formed distinct, sustainable vessel networks [49].
3.2. Microfluidics
As technology advances, techniques like microfluidics fabrication allows cells to be cultured with external factors including flow and electrical and mechanical stimulation to better mimic physiological conditions [50–53]. These factors play a major role in lymphatic vessel development and valve formation and has been implicated in hyperplasia in tumors [54]. Therefore, it is important to incorporate these factors in in vitro models.
Microfluidic systems have helped to highlight the role of flow in vessel formation. For example, Bonvin et al. utilized a multichambered microfluidic system that exposed LECs to flow through the chamber and was able to cause lymphatic capillary morphogenesis [55]. This system was able to be imaged in real-time and allowed for long-term culturing. In Choi et al., flow was used to show that shear stress, which is tangential to the flow, causes an increase in lymphatic sprouting [56]. Kim et al. used flow, biochemical cues, and stromal-endothelial interactions to cause vessel sprouting. While flow serves as the main determiner of lymphangiogenic responses, mechanical cues and pro-lymphangiogenic factors help to provide a robust response [57]. These models helped to show that flow plays a vital role in promoting and regulating lymphatic vessel sprouting. The next step in microfluidic improvements was to add cyclic flow. Fathi et al. showed that cells grown in cyclic flow are able to produce more physiologically relevant levels of interleukin 8 (IL-8) and tumor necrosis factor alpha (TNF-α) and have higher rates of LEC proliferation compared to cells grown in static conditions [58]. Additionally, Sabine et al. showed that shear stress due to oscillatory flow works cooperatively with FOXC2, a transcription factor that affects the organization of endothelial cytoskeleton, to maintain LEC quiescence and stabilize collecting vessels [59]. These models improved the understanding of how cyclic flow affects LECs and could lead to more physiologically relevant models in the future.
Next, signaling molecules were integrated into microfluidic systems. Nandagopal et al demonstrated that CCR7 ligands, known as CCL21 and CCL19, formed gradients in a microfluidic channel. They showed that CCL21, not CCL19, causes chemotaxis of T cells from peripheral human blood and CCL19 can cause a repulsive migration response [60]. This indicates CCL19 and CCL21 work together to cause migration. While Haessler et al, showed that DCs have a preference towards CCL21, but they need two times the amount of CCL19 to overcome this preference [61]. In Hoerning et al., the expression of CXC chemokine receptor 3 (CXCR3) on a subset of regulatory T cells (Tregs) and its role in migration was assessed [62]. In this case, a microfluidic device was used to assess chemotaxis and directional persistence, and Tregs were able to be measured at the single cell resolution.
Microfluidic models also offer real-time immunophenotyping via integrated biosensors, which could offer further insight into the types of immune cells that interact with the lymphatic system. Rodriguez-Moncayo et al. aimed to create an automated system that could assess cytokine secretions from a defined number of immune cells [63]. Immune cells were captured in microwell plates, stimulated with doses of lipopolysaccharide and phorbol 12-myristate 13-acetate–ionomycin, and the secretion of IL-8 and TNF-α was characterized. This model demonstrated that microfluidic devices can be used to monitor and quantify cytokine production, which could aid in both disease diagnosis and lymphatic studies.
Co-cultures can be integrated within microfluidic models, which allows cell-cell interactions and biophysical factors to be incorporated. Sato et al. used a co-culture of BECs and LECs in a microfluidic device to examine the vascular permeability of lymphatic and blood vessels [64]. This system demonstrated a more physiologically relevant model of the lymphatic-blood vessel interface. Additionally, microfluidic devices can be used to systematically study complex lymphatic environments, such as the tumor microenvironment. To further understand how altered ECM density in the tumor microenvironment effects lymphatic vessels, a microfluidic device with lymphatic vessels formed in a collagen type I matrix was created (Figure 3C–D). Higher ECM density, which mimicked cancerous breast tissue, caused lymphatic vessels to undergo more morphological changes, secrete higher amounts of pro-inflammatory cytokines, and display more leaky junctions, indicating that the ECM has a major effect on lymphatic vessel functionality [65]. Birmingham et al. modeled the SCS to determine the effects of LN remodeling on metastasis. This model showed that alterations to flow profiles, presentation of adhesive ligands, and monocytic cells contributed to the cell adhesions which are relevant to lymphatic metastasis [66].
Several advantages to microfluidic systems are that they can more accurately mimic 3-D microenvironments, can be used in high-throughput formats, have continuous monitoring and feedback capabilities, and consume low volumes of reagents. Unfortunately, microfluidic systems can be relatively expensive, difficult to image, and the versatility of biochemical gradients are limited by diffusion [67]. Biomaterials have already been integrated into some microfluidic systems. Various biomaterials, including collagen and fibrin, have been used as scaffolds to mimic microenvironments or support vessel formation [55,57,65]. Biomaterials like poly(dimethylsiloxane) (PDMS) have also been used to micropattern microfluidic chips [64].
3.3. Animal Models
As a result of the numerous types of interactions between the immune and lymphatic systems, there are many animal models that have been developed to study specific mechanisms. Due to the broad scope, we have selected just a few models to review below.
Particularly in the case of cancer metastasis, the role of lymphatics remains unclear as they can deliver immune cells to the tumor microenvironment but can also provide a route of metastasis. In a mouse melanoma model, the interaction between lymphangiogenesis and immunotherapy was investigated, and VEGF-C was found to attract naïve T cells which improved the therapeutic response [68].
A common mouse model employed for studies involving lymphatic vessel transport is the K14-VEGFR3-Ig transgenic mouse which lacks dermal lymphatics [69]. Utilizing this model, the individual roles of blood vessels and lymphatic vessels in immune trafficking or therapeutic delivery can be investigated in the absence of dermal lymphatic vessels. Due to this lymphatic vessel deficiency, lymphedema results and a lack of macromolecular transport in the dermis occurs [69]. Additionally, K14-VEGFR3-Ig mice exhibit decreased trafficking of solutes and DCs from the skin to the dLNs [70].
Utilizing this K14-VEGFR3-Ig mouse model, the role of lymphatic drainage on humoral immunity and peripheral tolerance was investigated. While effector T cell immunity was only transiently and negligibly affected by the absence of dermal lymphatics, the transgenic mice produced lower antibody titers upon dermal immunization. Additionally, multiple signs of autoimmunity were observed in one-year-old mice [70].
Beyond the dermis, K14-VEGFR3-Ig mice have also been used to study the role of lymphatic vessels and immune cells in a traumatic brain injury (TBI) model. The recent discovery of meningeal lymphatic vessels has sparked numerous investigations about the role of lymph in neurological conditions. After sustaining a TBI, K14-VEGFR3-Ig mice had a significantly reduced population of infiltrating CD4+ T lymphocytes which suggests that these meningeal lymphatic vessels are pertinent for a proper neuro-immune response that is hypothesized to be principally mediated by resident memory CD8+ T cells [71].
While animal models can capture the complexity of the immune response and provide a more advanced system compared to in vitro models, there are several challenges including the added complexity of systemic responses and phylogenetic discrepancies between laboratory animals and humans. These challenges highlight how co-cultures and microfluidic models could supplement animal models. Used together in a combinatorial approach, these three model systems provide the ability to investigate specific interactions in a defined system, use patient-specific cells in a physiologically relevant model, and study the aggregate host response.
4. Pharmaceutical Uptake in the Lymphatic System
The anatomy of the lymphatic system must be considered when designing therapeutics, and multiple factors dictate how molecules or therapeutics will interact with the lymphatic circulation. Additionally, the route of administration and desired pharmacokinetics must be considered in the design.
4.1. Factors influencing drug uptake via the lymphatics
Due to the varied anatomy and organ-specific structure of lymphatic vessels, ranging from capillaries with discontinuous, button-like junctions to collecting vessels with tighter, zipper-like junctions, multiple factors must be considered for therapeutic delivery, including the particle size, molecular weight, surface charge, and hydrophobicity (Figure 4A). When delivering pharmaceuticals via nanoparticles, the particle size will affect both the rate of uptake and retention. While particles larger than 100 nm can eventually be taken up by the lymphatic system, 10–100 nm is the optimal size range for subcutaneous administration [40,72]. When delivering soluble pharmaceuticals subcutaneously, the molecular weight will influence the degree of uptake via the lymphatic system, with molecules under 1 kDa being easily absorbed by blood capillaries, versus molecules exceeding 16 kDa will be mostly absorbed by the lymphatic system surrounding the local injection site [40]. The interstitial matrix has a net negative charge, which influences how particles are taken up by the lymphatics. Compared to positively charged liposomes, negatively charged liposomes exhibited increased lymphatic uptake, and interestingly, neutral particles had the lowest uptake [73]. Furthermore, highly negatively charged particles also demonstrated increased retention times in LNs [74]. Additionally, lymphatic uptake can be modified by altering the hydrophobicity of particles, as the hydrophobicity is mainly responsible for phagocytosis [40].
Figure 4. Multiple factors must be considered when designing therapeutics for immunomodulation via the lymphatic systems.
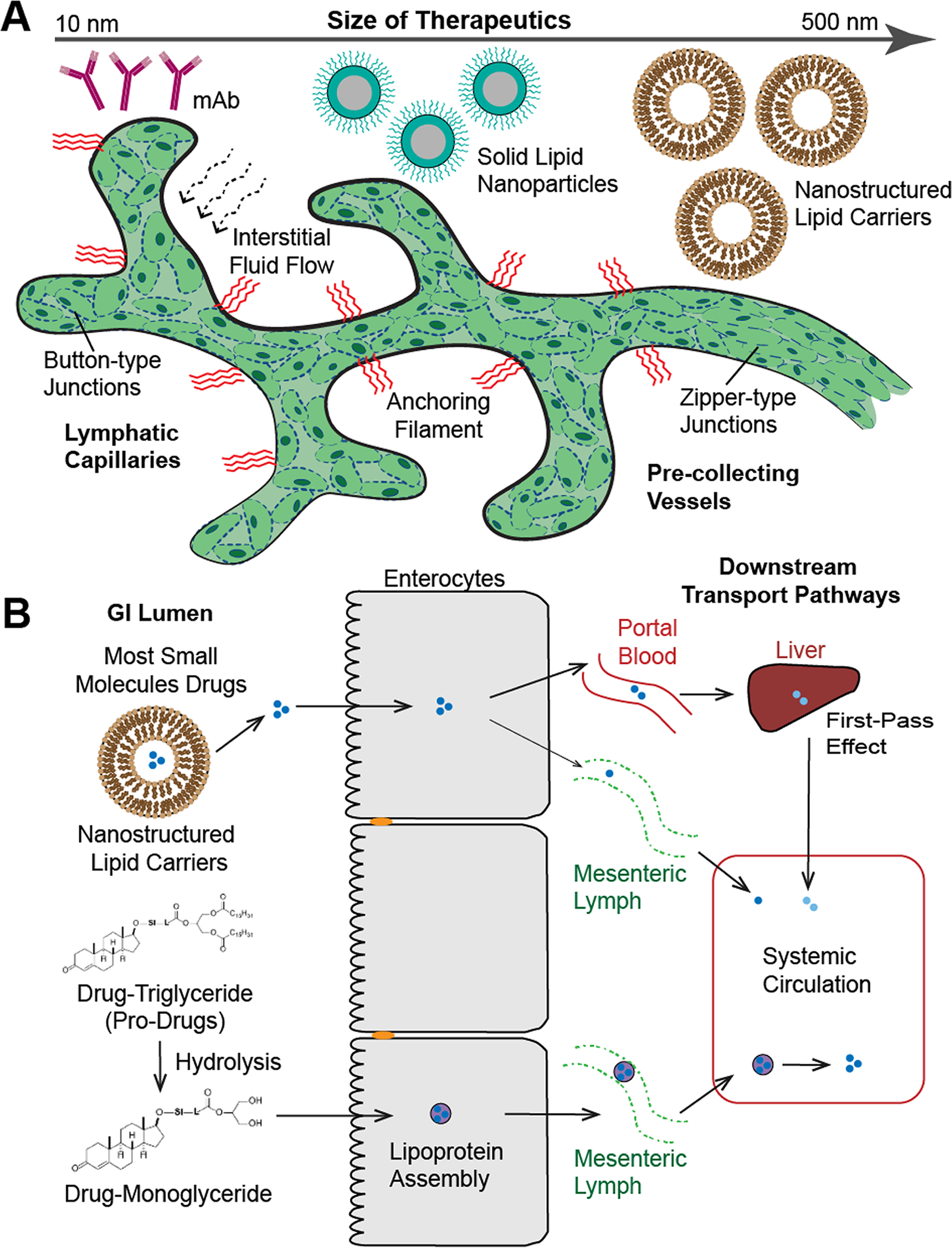
(A) Different types and sizes of therapeutics, such as monoclonal antibodies (mAb, 10 nm), solid lipid nanoparticles (200 nm), and nanostructured lipid carries (500 nm) that can be used to target the lymphatic system. As a reference, chylomicrons up to 1,000nm in diameter can be transported across the lacteals [92]. The specific structure of the lymphatic vessels being targeted must be considered, as their structure is organ-specific and vary in permeability depending on the types of junctions (button-type and zipper-type junctions), which will influence the possible biomaterials used for therapeutic delivery. (B) Schematic diagram of the different mechanisms of transport pathways following oral drug or pro-drug administration. The intestinal transport of lipid-based formulations (nanostructured lipid carries) through blood (major) and lymphatic circulation (minor). Pro-drug formulation as a drug-triglyceride undergoes hydrolysis into drug-monoglyceride, which will be further assembled into lipoprotein to enter the mesenteric lymph. Therefore, the drug can enter the systemic circulation by avoiding the first pass metabolism in the liver. Illustration in panel B was adapted with permission from [79].
4.2. Routes of administration via the lymphatic system
A unique aspect of drug delivery via the lymphatic system is that first-pass metabolism, which is typically associated with oral administration and results in reduced systemic concentrations, is avoided which is advantageous for therapeutics with lower bioavailability. Additionally, delivery through the lymphatic system can be used to combat negative outcomes associated with systemic administration, particularly for chemotherapeutics and other cytotoxic agents, which include non-specificity, drug resistance, and severe toxicity [40]. While systemic drug administration is the predominant delivery route used in both preclinical models and human patients, the tumor microenvironment and tumor draining lymph nodes (TdLNs) are poorly accessed, there is decreased targeting, and decreased circulation time which all raise challenges for this route of administration [75,76]. Instead, by targeting delivery to the lymphatics in a rat model, localized tissue concentrations of chemotherapeutics were increased and also resulted in reduced organ toxicity and nephrotoxicity [77]. Alternative routes of administration include intestinal, subcutaneous, pulmonary, and loco-regional delivery, as well as targeting the dLNs. Advantages and applications of each delivery route are discussed in the strategies for lymphatic drug delivery section of this review.
5. Strategies for lymphatic drug delivery
5.1. Gastrointestinal delivery
Although the gastrointestinal (GI) tract is a preferred route for drug delivery, therapeutics administered by this route are subjected to pre-systemic hepatic metabolism. Additionally, the drug’s solubility, the pH of the GI tract, and the amount of time in the GI tract all affect drug bioavailability and may lower availability. These challenges highlight areas that can be remedied by targeting drug delivery to the intestinal lymphatic system. Due to the overlapping and gapped structure of the lymphatic capillaries, macromolecular targeting to the lymphatic system through intestinal transport is possible. In the gut-associated lymphoid tissue, lymphoid follicles form Peyer’s patches and also provide an entry point into the lymphatic system [40]. Lipophilic drugs have been shown to be absorbed via intestinal lymphatics following oral administration, and this transport method can considerably improve the drug’s metabolic profile compared to transport via the portal system [78]. One method to direct drug absorption through the lymphatics instead of the portal system is by using a pro-drug formulation. A pro-drug formulation as a drug-triglyceride undergoes hydrolysis into a drug-monoglyceride, which is then further assembled into a lipoprotein to enter the mesenteric lymph. Therefore, the drug can enter the systemic circulation by avoiding the first pass metabolism in the liver (Figure 4B) [79].
By incorporating a drug within a nanoparticle, up to 500 nm in diameter, targeted delivery to the intestines via lymphatic uptake can be achieved. Compared to intravenous administration of a drug alone, drug-containing nanoparticles delivered to the intestines demonstrated a 21-fold increase in drug bioavailability, a 30-fold increase in the elimination half-life, and lower distribution to the heart, lungs, spleen, and kidneys. These results highlight how targeted delivery to the intestines through the lymphatic system could both be used as an extended-release drug delivery system, as well as a strategy to reduce toxicity [80].
5.2. Subcutaneous delivery
In the case of subcutaneous delivery, drug accumulation occurs at the site of administration, there is a sustained release, and increased absorption [40]. Compared to intraperitoneal (IP) or intravenous (IV) drug administration in a lymphoma mouse model, subcutaneous administration of drug-containing nanoparticles demonstrated an 8-fold and 59-fold higher drug uptake, respectively. Additionally, lower drug uptake in the lungs, liver, and spleen were observed, which resulted in longer circulation of the nanoparticles. Despite drug uptake initially being slow following subcutaneous administration, uptake continued over time and demonstrated the possibility of controlled release therapy by this route [81].
5.3. Pulmonary delivery
For particles up to 200 nm in diameter, alveolar clearance involves the lymphatic system and presents the possibility of targeted drug delivery via the pulmonary lymphatics [82]. This route of administration could be particularly advantageous for treating certain diseases, such as small cell lung carcinoma, that disseminate through the pulmonary system. Additionally, pulmonary administration avoids first-pass metabolism, is noninvasive, and allows for increased local concentrations of the drug [40]. The nanoparticle size can also be adjusted to target specific regions for drug deposition within the lungs. When drug-loaded nanoparticles were administered to mice with non-small cell lung cancer via nebulization to target delivery to the pulmonary lymphatics, a 20-fold reduction in IC50 was observed, compared to intravenous administration of the drug alone, as well as reduced toxicity [83].
5.4. Loco-regional delivery
Compared to conventional systemic immune checkpoint blockade (ICB) therapy, which has variable response rates and off-target toxicity, intraturmoral or intradermal locoregional delivery of monoclonal antibodies (mAbs) demonstrated improved T cell responses [75]. While systemic drug administration is the predominant delivery method in the lab and clinic, the tumor microenvironment and TdLNs are poorly accessed [84]. By targeting delivery of mAbs to the TdLNs, improved immunomodulation within the TdLNs was achieved. As a result, less doses were required, anti-tumor immunity was improved, and toxicity was potentially limited [75]. This strategy can be particularly effective for locally advanced cancers, as it allows for higher local concentrations of chemotherapeutics supplemented with lower systemic levels to treat distant metastases [77]. When molecules comparable in size to mAbs were injected in the interstitium of peripheral tissues, the initial lymphatics were responsible for clearing the compound and caused accumulation of the compound in the dLNs, which highlights one method for achieving this targeted delivery [85].
6. Drug Screening and Disease Prediction
Despite recent advancements regarding the discovery of biomarkers for cancer detection, these biomarkers have low specificity and typically very low concentrations in plasma which limits their detection in liquid biopsies. While noninvasive to the patient, these limitations of liquid biopsy prevent detecting metastatic spread during the early stages. Alternatively, samples could be acquired from the tumor-draining lymphatic vessels since biomarkers preferentially drain to lymphatic vessels before becoming diluted in the blood circulation, which should provide an enriched concentration and may allow for early detection [86].
Circulating extracellular vesicles (EVs), specifically exosomes, contain tumor-relevant factors and have emerged as potential biomarkers [86]. EVs are transported from the periphery to draining LNs via lymphatic vessels [87], and when fluorescent EVs were injected into mice, EVs were absent from the surrounding blood capillaries but found in the draining lymphatic vessels which highlights the important role lymphatic vessels have regarding EV circulation. Analysis of postoperative lymphatic exudate from patients with metastatic melanoma revealed that tumor-derived biomarkers, particularly EVs which contained melanoma-associated miRNAs, were significantly enriched [86]. Lymph contains tissue-derived self-antigens which reflect its origin [88], and it also mirrors the tissue inflammatory signature [86]. In the sampled lymphatic exudate, these specific protein signatures distinguished various stages of metastatic spread, posing the potential to be used as a novel detection method to characterize the disease stage and predict therapeutic responses [86].
7. Challenges and Future Outlooks
While the development of new models and methods has improved the field of lymphatic system modulation and delivery, there are still challenges that need to be addressed. Firstly, there are drawbacks with both natural and synthetic biomaterials. Natural biomaterials have low risk of biotoxicity and are bioactive. However, they are usually more prone to mechanical failure, can lack biostability, and can be immunogenic [89]. On the other hand, synthetic biomaterials can possess more uniformity, and have controllable mechanical properties, but they are more likely to cause an inflammatory response, less likely to integrate with tissues, and there are challenges with developing a synthetic biomaterial that can mimic the mechanical cues provided by naturally occurring materials [89,90]. Secondly, it is difficult to release lymphangiogenic factors at a physiologically relevant, sustained rate with hydrogels which could limit the feasibility of hydrogels for long term in vivo use. One solution that has been utilized for angiogenesis is the delivery of gene therapies via hydrogels that would cause cells to release the factors. While there are plenty of instances of gene therapies being used in the cardiovascular system and many studies that utilize lymphangiogenic genes, there is little research on controlled delivery of lymphatic gene therapy [90]. Thirdly, many current in vitro models are not as physiologically relevant as they could be. Current models tend to focus on either the lymphatic or immune system, but there are not many models that do both. Combining these areas could result in more information about interactions between immune cells, such as migrating T cells, and the lymphatic system. Additionally, there are challenges with allowing for a reversible immune response in these models, which would mimic the dynamic exchange of cells and materials seen in vivo [53].
Thus far most studies have focused on lymphatic capillaries or LNs, but have excluded the lymphatic muscle cells, pericytes, and valves needed to form collecting vessels [23,90]. As the pumping mechanism of the vasculature is vital to collecting vessel formation, “artery-on-a-chip” style microfluidic devices have the potential to be leveraged [91]. Additionally, there is an interest in developing biomaterials to mimic the dynamic signaling seen in diseased tissue. In the event of injury, natural ECM and proteases provide signals and soluble factors that can direct endothelial migration to support tissue regeneration and ECM remodeling. This mechanism can be utilized for revascularization and could potentially modulate lymphatics [90]. Given these advancements and future potential, biomaterials demonstrate the ability to be used for both model systems and as delivery platforms for immunomodulation applications involving the lymphatic system.
Table 1:
Summary of Lymphatic models
| Model | Cells/Ligands Used | Biomaterials Used | Result | Ref. |
|---|---|---|---|---|
| Co-Culturing | LECs & Fibroblasts | Fibroblasts | Generation of lymphatic vessels without the introduction of exogenous factors | [45] |
| Tumor Cells, LECs, & CCR7 | Collagen | Chemokine secretion directs tumor cells with flow | [44] | |
| T Cells & LECs | N/A | T cell proliferation and tolerogenic properties expressed by LN-LECs | [41] | |
| Fibroblasts & DCs | N/A | Allowed for DCs to migrate into the lymphatic system | [42] | |
| BECs, LECs, and ASCs | Fibrin | The BECs and LECs formed distinct, sustainable vessel networks | [49] | |
| Microfluidics | LECs | PDMS and Polyethylene | Caused lymphatic capillary morphogenesis | [55] |
| HMVEC | PDMS and Fibrinogen | Mechanical cues play a vital role in lymphangiogenesis | [57] | |
| LECs & BECs | Polyethylene Terephthalate (PET), PDMS, and Fibronectin | Established a model to assess permeability and lymphatic return rate | [64] | |
| LECs | PDMS and Fibronectin | Cells grown in flow can produce more physiologically relevant levels of IL-8 and TNF-α and have higher rates of LEC proliferation compared to cells in static conditions | [58] | |
| LECs | Fibronectin | Oscillatory shear stress in conjunction with FOXC2 can maintain LEC quiescence and stabilize collecting vessels | [59] | |
| T Cells | Anti-CD3 mAbs | The homing receptor CXCR3 is expressed on different subsets of T cells and is involved in their recruitment to peripheral inflammation sites | [62] | |
| Monocytes | PDMS | Developed a model to measure cytokine secretion | [63] | |
| T Cells, CCL21, & CCL19 | PDMS | CCL19 and CCL21 work together to cause T cell migration | [60] | |
| DCs, CCL21, & CCL19 | Agarose | CCL21 is preferred over CCL19 for DCs chemotaxis | [61] | |
| LECs & Metastatic Breast Cancer Cells | Collagen Type I | ECM is an important factor in the tumor microenvironment that can affect the lymphatic vessel functionality. | [65] | |
| Monocytes | PDMS, ICAM, and VCAM | Alterations to flow profiles, presentation of adhesive ligands, and monocytic cells contribute to cell adhesion relevant to LN invasion prevalent to lymphatic metastasis. | [66] | |
| Animal | K14-VEGFR3-Ig mice | Soluble VEGFR-3 | Dermal lymphatics are absent in these transgenic mice which results in impaired drug uptake and cell trafficking. | [69] |
| OT-1 RAG-1−/− and BrafV600E/Pten−/− mice | Anti-VEGFR3 antibody, CpG-B peptide, and Trp-2-peptide conjugated NPs | VEGF-C signaling enhanced the response to immunotherapy in melanoma model | [68] | |
| Yucatan minipigs | BioBridge, aligned collagen fibers | Increased lymphatic vessel density near site of implant, improved bioimpedance ratio suggesting restoration of lymphatic drainage | [36] |
Statement of Significance.
The lymphatic system plays an integral part in regulating immune cells trafficking and the transport of macromolecules. However, its influence on disease progression and drug uptake is understood less than that of the vascular system. This review article focuses on recent progresses in biomaterials to investigate the lymphatic system and to provide novel understanding into complex disease states. Insight gained from these mechanistic studies can be further used to design innovative biomaterials to modulate the immune system, improve drug delivery, and promote tissue regeneration. Finally, a number of challenges in adopting biomaterials for immunomodulation and future perspectives are discussed.
ACKNOWLEDGMENTS
We acknowledge support from the University of Notre Dame through “Advancing our Vision” initiative, the Harper Cancer Research Institute - American Cancer Society Institutional Research Grant (IRG-17-182-04), American Heart Association through Career Development Award (19-CDA-34630012 to D.H.-P.), National Science Foundation (2047903 to D.H.-P.), and from the Walther Cancer Foundation (Pre-doctoral Fellowship to L.A.). We thank Thomas Loomis (Engineering Graphics and Publications at the University of Notre Dame) for his assistance with the graphical contents. This publication was made possible, with support from the Indiana Clinical and Translational Science Institute (I-CTSI) funded, in part by Grant Number ULITR001108 from the NIH for Advancing Translational Sciences, Clinical and Translational Science Awards.
FUNDING
ACS IRG-17-182-04
19-CDA-34630012
NIH ULITR001108
NSF-CBET-2047903
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Petrova TV, Koh GY, Biological functions of lymphatic vessels, Science. 369 (2020). 10.1126/science.aax4063. [DOI] [PubMed] [Google Scholar]
- [2].Goswami AK, Khaja MS, Downing T, Kokabi N, Saad WE, Majdalany BS, Lymphatic Anatomy and Physiology, Semin. Interv. Radiol 37 (2020) 227–236. 10.1055/s-0040-1713440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hunter MC, Teijeira A, Halin C, T Cell Trafficking through Lymphatic Vessels, Front. Immunol 7 (2016). 10.3389/fimmu.2016.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Liao S, von der Weid PY, Lymphatic system: An active pathway for immune protection, Semin. Cell Dev. Biol 38 (2015) 83–89. 10.1016/j.semcdb.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jackson DG, Hyaluronan in the lymphatics: The key role of the hyaluronan receptor LYVE-1 in leucocyte trafficking, Matrix Biol. 78–79 (2019). 10.1016/j.matbio.2018.02.001. [DOI] [PubMed] [Google Scholar]
- [6].Wiig H, Swartz MA, Interstitial Fluid and Lymph Formation and Transport: Physiological Regulation and Roles in Inflammation and Cancer, Physiol. Rev 92 (2012). 10.1152/physrev.00037.2011. [DOI] [PubMed] [Google Scholar]
- [7].Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, McDonald DM, Functionally specialized junctions between endothelial cells of lymphatic vessels, J. Exp. Med 204 (2007). 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Park C, Hwang I-Y, Sinha RK, Kamenyeva O, Davis MD, Kehrl JH, Lymph node B lymphocyte trafficking is constrained by anatomy and highly dependent upon chemoattractant desensitization, Blood. 119 (2012). 10.1182/blood-2011-06-364273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Braun A, Worbs T, Moschovakis GL, Halle S, Hoffmann K, Bölter J, Münk A, Förster R, Afferent lymph–derived T cells and DCs use different chemokine receptor CCR7–dependent routes for entry into the lymph node and intranodal migration, Nat. Immunol 12 (2011). 10.1038/ni.2085. [DOI] [PubMed] [Google Scholar]
- [10].Card CM, Yu SS, Swartz MA, Emerging roles of lymphatic endothelium in regulating adaptive immunity, J. Clin. Invest 124 (2014). 10.1172/JCI73316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sixt M, Kanazawa N, Selg M, Samson T, Roos G, Reinhardt DP, Pabst R, Lutz MB, Sorokin L, The Conduit System Transports Soluble Antigens from the Afferent Lymph to Resident Dendritic Cells in the T Cell Area of the Lymph Node, Immunity. 22 (2005). 10.1016/j.immuni.2004.11.013. [DOI] [PubMed] [Google Scholar]
- [12].Liao S, Padera TP, Lymphatic Function and Immune Regulation in Health and Disease, Lymphat. Res. Biol 11 (2013). 10.1089/lrb.2013.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Frye M, Taddei A, Dierkes C, Martinez-Corral I, Fielden M, Ortsäter H, Kazenwadel J, Calado DP, Ostergaard P, Salminen M, He L, Harvey NL, Kiefer F, Mäkinen T, Matrix stiffness controls lymphatic vessel formation through regulation of a GATA2-dependent transcriptional program, Nat. Commun 9 (2018) 1511. 10.1038/s41467-018-03959-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Alderfer L, Russo E, Archilla A, Coe B, Hanjaya-Putra D, Matrix stiffness primes lymphatic tube formation directed by vascular endothelial growth factor-C, FASEB J. (2021). 10.1096/fj.202002426RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Abdalla S, Makhoul G, Duong M, Chiu RCJ, Cecere R, Hyaluronic acid-based hydrogel induces neovascularization and improves cardiac function in a rat model of myocardial infarction, Interact. Cardiovasc. Thorac. Surg 17 (2013) 767–772. 10.1093/icvts/ivt277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ratner B, Hoffman A, Schoen F, Lemons J, Biomaterials Science, 2013.
- [17].Marei W, Ghafari F, Fouladi-Nashta A, Role of hyaluronic acid in maturation and further early embryo development of bovine oocytes, Theriogenology. 78 (2012) 670–677. [DOI] [PubMed] [Google Scholar]
- [18].Luo Y, Kirker K, Prestwich G, Cross-linked hyaluronic acid hydrogel films: new biomaterials for drug delivery, J. Controlled Release 69 (2000). [DOI] [PubMed] [Google Scholar]
- [19].Greco R, Iocono J, Ehrlich H, Hyaluronic acid stimulates human fibroblast proliferation within a collagen matrix, J. Cell. Physiol 177 (1998). [DOI] [PubMed] [Google Scholar]
- [20].Kim J, Kim I, Cho T, Lee K, Hwang S, Tae G, Bone regeneration using hyaluronic acid-based hydrogel with bone morphogenic protein-2 and human mesenchymal stem cells, Biomaterials. 28 (2007). [DOI] [PubMed] [Google Scholar]
- [21].Burdick JA, Prestwich GD, Hyaluronic Acid Hydrogels for Biomedical Applications, Adv. Mater 23 (2011) H41–H56. 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Banerji S, Ni J, Wang S-X, Clasper S, Su J, Tammi R, Jones M, Jackson DG, LYVE-1, a New Homologue of the CD44 Glycoprotein, Is a Lymph-specific Receptor for Hyaluronan, J. Cell Biol 144 (1999) 789 LP – 801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Alderfer L, Wei A, Hanjaya-Putra D, Lymphatic Tissue Engineering and Regeneration, J. Biol. Eng 12 (2018) 32. 10.1186/s13036-018-0122-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hanjaya-Putra D, Bose V, Shen Y-I, Yee J, Khetan S, Fox-Talbot K, Steenbergen C, Burdick JA, Gerecht S, Controlled activation of morphogenesis to generate a functional human microvasculature in a synthetic matrix, Blood. 118 (2011) 804 LP – 815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hanjaya-Putra D, Yee J, Ceci D, Truitt R, Yee D, Gerecht S, Vascular endothelial growth factor and substrate mechanics regulate in vitro tubulogenesis of endothelial progenitor cells, J. Cell. Mol. Med 14 (2010) 2436–2447. 10.1111/j.1582-4934.2009.00981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B, CD44 is the principal cell surface receptor for hyaluronate, Cell. 61 (1990) 1303–1313. 10.1016/0092-8674(90)90694-A. [DOI] [PubMed] [Google Scholar]
- [27].Johnson LA, Banerji S, Lagerholm BC, Jackson DG, Dendritic cell entry to lymphatic capillaries is orchestrated by CD44 and the hyaluronan glycocalyx, Life Sci. Alliance 4 (2021) e202000908. 10.26508/lsa.202000908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].McDonald B, Kubes P, Interactions between CD44 and hyaluronan in leukocyte trafficking, Front. Immunol 6 (2015) 1–6. 10.3389/fimmu.2015.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bollyky PL, Evanko SP, Wu RP, Potter-Perigo S, Long SA, Kinsella B, Reijonen H, Guebtner K, Teng B, Chan CK, Braun KR, Gebe JA, Nepom GT, Wight TN, Th1 cytokines promote T-cell binding to antigen-presenting cells via enhanced hyaluronan production and accumulation at the immune synapse, Cell. Mol. Immunol 7 (2010) 211–220. 10.1038/cmi.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Vega SL, Kwon MY, Song KH, Wang C, Mauck RL, Han L, Burdick JA, Combinatorial hydrogels with biochemical gradients for screening 3D cellular microenvironments, Nat. Commun 9 (2018) 1–10. 10.1038/s41467-018-03021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Abdalla S, Makhoul G, Duong M, Chiu RCJ, Cecere R, Hyaluronic acid-based hydrogel induces neovascularization and improves cardiac function in a rat model of myocardial infarction, Interact Cardiovasc Thorac Surg. 17 (2013) 767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Helm C-L, Zisch A, Swartz MA, Engineered Blood and Lymphatic Capillaries in 3-D VEGF-Fibrin-Collagen Matrices with Interstitial Flow, Biotechnol. Bioeng 96 (2006). 10.1002/bit. [DOI] [PubMed] [Google Scholar]
- [33].Marino D, Luginbühl J, Scola S, Meuli M, Reichmann E, Bioengineering: Bioengineering dermo-epidermal skin grafts with blood and lymphatic capillaries, Sci. Transl. Med 6 (2014). 10.1126/scitranslmed.3006894. [DOI] [PubMed] [Google Scholar]
- [34].Li T, Wang G, Tan Y, Wang H, Inhibition of Lymphangiogenesis of Endothelial Progenitor Cells with VEGFR-3 siRNA Delivered with PEI-alginate Nanoparticles, Int. J. Biol. Sci 10 (2014) 160–170. 10.7150/ijbs.6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Reddy ST, Rehor A, Schmoekel HG, Hubbell JA, Swartz MA, In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles, J. Controlled Release 112 (2006) 26–34. 10.1016/j.jconrel.2006.01.006. [DOI] [PubMed] [Google Scholar]
- [36].Hadamitzky C, Zaitseva TS, Bazalova-Carter M, Paukshto MV, Hou L, Strassberg Z, Ferguson J, Matsuura Y, Dash R, Yang PC, Kretchetov S, Vogt PM, Rockson SG, Cooke JP, Huang NF, Aligned nanofibrillar collagen scaffolds – Guiding lymphangiogenesis for treatment of acquired lymphedema, Biomaterials. 102 (2016) 259–267. 10.1016/j.biomaterials.2016.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Campbell KT, Hadley DJ, Kukis DL, Silva EA, Alginate hydrogels allow for bioactive and sustained release of VEGF-C and VEGF-D for lymphangiogenic therapeutic applications, PloS One. 12 (2017) e0181484–e0181484. 10.1371/journal.pone.0181484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Najibi AJ, Mooney DJ, Cell and tissue engineering in lymph nodes for cancer immunotherapy, Adv. Drug Deliv. Rev (2020). 10.1016/j.addr.2020.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bauleth-Ramos T, Shih TY, Shahbazi MA, Najibi AJ, Mao AS, Liu D, Granja P, Santos HA, Sarmento B, Mooney DJ, Acetalated Dextran Nanoparticles Loaded into an Injectable Alginate Cryogel for Combined Chemotherapy and Cancer Vaccination, Adv. Funct. Mater 29 (2019) 1–10. 10.1002/adfm.201903686. [DOI] [Google Scholar]
- [40].Khan AA, Mudassir J, Mohtar N, Darwis Y, Advanced drug delivery to the lymphatic system: Lipid-based nanoformulations, Int. J. Nanomedicine 8 (2013) 2733–2744. 10.2147/IJN.S41521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cohen JN, Tewalt EF, Rouhani SJ, Buonomo EL, Bruce AN, Xu X, Bekiranov S, Fu Y-X, Engelhard VH, Tolerogenic Properties of Lymphatic Endothelial Cells Are Controlled by the Lymph Node Microenvironment, PLoS ONE. 9 (2014). 10.1371/journal.pone.0087740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Khosravi-Maharlooei M, Pakyari M, Jalili RB, Salimi-Elizei S, Lai JCY, Poormasjedi-Meibod M, Kilani RT, Dutz J, Ghahary A, Tolerogenic effect of mouse fibroblasts on dendritic cells, Immunology. 148 (2016). 10.1111/imm.12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Triacca V, Güç E, Kilarski WW, Pisano M, Swartz MA, Transcellular Pathways in Lymphatic Endothelial Cells Regulate Changes in Solute Transport by Fluid Stress, Circ. Res 120 (2017). 10.1161/CIRCRESAHA.116.309828. [DOI] [PubMed] [Google Scholar]
- [44].Shields JD, Fleury ME, Yong C, Tomei AA, Randolph GJ, Swartz MA, Autologous Chemotaxis as a Mechanism of Tumor Cell Homing to Lymphatics via Interstitial Flow and Autocrine CCR7 Signaling, Cancer Cell. 11 (2007). 10.1016/j.ccr.2007.04.020. [DOI] [PubMed] [Google Scholar]
- [45].Gibot L, Galbraith T, Kloos B, Das S, Lacroix DA, Auger FA, Skobe M, Cell-based approach for 3D reconstruction of lymphatic capillaries in vitro reveals distinct functions of HGF and VEGF-C in lymphangiogenesis, Biomaterials. 78 (2016). 10.1016/j.biomaterials.2015.11.027. [DOI] [PubMed] [Google Scholar]
- [46].Vis MAM, Ito K, Hofmann S, Impact of Culture Medium on Cellular Interactions in in vitro Co-culture Systems, Front. Bioeng. Biotechnol 8 (2020) 911. 10.3389/fbioe.2020.00911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Battiston KG, Cheung JWC, Jain D, Santerre JP, Biomaterials in co-culture systems: Towards optimizing tissue integration and cell signaling within scaffolds, Biomaterials. 35 (2014). 10.1016/j.biomaterials.2014.02.023. [DOI] [PubMed] [Google Scholar]
- [48].Bogdanowicz DR, Lu HH, Studying cell-cell communication in co-culture, Biotechnol. J 8 (2013). 10.1002/biot.201300054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Knezevic L, Schaupper M, Mühleder S, Schimek K, Hasenberg T, Marx U, Priglinger E, Redl H, Holnthoner W, Engineering Blood and Lymphatic Microvascular Networks in Fibrin Matrices, Front. Bioeng. Biotechnol 5 (2017). 10.3389/fbioe.2017.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bhatia SN, Ingber DE, Microfluidic organs-on-chips, Nat. Biotechnol 32 (2014) 760–772. 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- [51].Naik N, Hanjaya-Putra D, Haller CA, Allen MG, Chaikof EL, Rapid homogeneous endothelialization of high aspect ratio microvascular networks, Biomed. Microdevices 17 (2015) 83. 10.1007/s10544-015-9990-5. [DOI] [PubMed] [Google Scholar]
- [52].Abaci HE, Shen Y-I, Tan S, Gerecht S, Recapitulating physiological and pathological shear stress and oxygen to model vasculature in health and disease, Sci. Rep 4 (2014) 4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kim S, Shah SB, Graney PL, Singh A, Multiscale engineering of immune cells and lymphoid organs, Nat. Rev. Mater 4 (2019) 355–378. 10.1038/s41578-019-0100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Planas-Paz L, Lammert E, Mechanical forces in lymphatic vascular development and disease, Cell. Mol. Life Sci 70 (2013). 10.1007/s00018-013-1358-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Bonvin C, Overney J, Shieh AC, Dixon JB, Swartz MA, A multichamber fluidic device for 3D cultures under interstitial flow with live imaging: Development, characterization, and applications, Biotechnol. Bioeng (2010). 10.1002/bit.22608. [DOI] [PubMed] [Google Scholar]
- [56].Choi D, Park E, Jung E, Seong YJ, Yoo J, Lee E, Hong M, Lee S, Ishida H, Burford J, Peti-Peterdi J, Adams RH, Srikanth S, Gwack Y, Chen CS, Vogel HJ, Koh CJ, Wong AK, Hong Y-K, Laminar flow downregulates Notch activity to promote lymphatic sprouting, J. Clin. Invest 127 (2017) 1225–1240. 10.1172/JCI87442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kim S, Chung M, Jeon NL, Three-dimensional biomimetic model to reconstitute sprouting lymphangiogenesis in vitro, Biomaterials. 78 (2016). 10.1016/j.biomaterials.2015.11.019. [DOI] [PubMed] [Google Scholar]
- [58].Fathi P, Holland G, Pan D, Esch MB, Lymphatic Vessel on a Chip with Capability for Exposure to Cyclic Fluidic Flow, ACS Appl. Bio Mater 3 (2020). 10.1021/acsabm.0c00609. [DOI] [PubMed] [Google Scholar]
- [59].Sabine A, Bovay E, Demir CS, Kimura W, Jaquet M, Agalarov Y, Zangger N, Scallan JP, Graber W, Gulpinar E, Kwak BR, Mäkinen T, Martinez-Corral I, Ortega S, Delorenzi M, Kiefer F, Davis MJ, Djonov V, Miura N, Petrova TV, FOXC2 and fluid shear stress stabilize postnatal lymphatic vasculature, J. Clin. Invest 125 (2015) 3861–3877. 10.1172/JCI80454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Nandagopal S, Wu D, Lin F, Combinatorial Guidance by CCR7 Ligands for T Lymphocytes Migration in Co-Existing Chemokine Fields, PLoS ONE. 6 (2011). 10.1371/journal.pone.0018183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Haessler U, Pisano M, Wu M, Swartz MA, Dendritic cell chemotaxis in 3D under defined chemokine gradients reveals differential response to ligands CCL21 and CCL19, Proc. Natl. Acad. Sci 108 (2011). 10.1073/pnas.1014920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hoerning A, Koss K, Datta D, Boneschansker L, Jones CN, Wong IY, Irimia D, Calzadilla K, Benitez F, Hoyer PF, Harmon WE, Briscoe DM, Subsets of human CD4+ regulatory T cells express the peripheral homing receptor CXCR3, Eur. J. Immunol 41 (2011). 10.1002/eji.201041095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Rodriguez-Moncayo R, Jimenez-Valdes RJ, Gonzalez-Suarez AM, Garcia-Cordero JL, Integrated Microfluidic Device for Functional Secretory Immunophenotyping of Immune Cells, ACS Sens. 5 (2020). 10.1021/acssensors.9b01786. [DOI] [PubMed] [Google Scholar]
- [64].Sato M, Sasaki N, Ato M, Hirakawa S, Sato K, Sato K, Microcirculation-on-a-Chip: A Microfluidic Platform for Assaying Blood- and Lymphatic-Vessel Permeability, PLOS ONE. 10 (2015) e0137301. 10.1371/journal.pone.0137301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Lugo-Cintrón KM, Ayuso JM, White BR, Harari PM, Ponik SM, Beebe DJ, Gong MM, Virumbrales-Muñoz M, Matrix density drives 3D organotypic lymphatic vessel activation in a microfluidic model of the breast tumor microenvironment, Lab. Chip 20 (2020). 10.1039/D0LC00099J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Birmingham KG, O’Melia MJ, Bordy S, Reyes Aguilar D, El-Reyas B, Lesinski G, Thomas SN, Lymph Node Subcapsular Sinus Microenvironment-On-A-Chip Modeling Shear Flow Relevant to Lymphatic Metastasis and Immune Cell Homing, IScience. 23 (2020). 10.1016/j.isci.2020.101751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Coluccio ML, Perozziello G, Malara N, Parrotta E, Zhang P, Gentile F, Limongi T, Raj PM, Cuda G, Candeloro P, Di Fabrizio E, Microfluidic platforms for cell cultures and investigations, Microelectron. Eng 208 (2019) 14–28. 10.1016/j.mee.2019.01.004. [DOI] [Google Scholar]
- [68].Fankhauser M, Broggi MAS, Potin L, Bordry N, Jeanbart L, Lund AW, Da Costa E, Hauert S, Rincon-Restrepo M, Tremblay C, Cabello E, Homicsko K, Michielin O, Hanahan D, Speiser DE, Swartz MA, Tumor lymphangiogenesis promotes T cell infiltration and potentiates immunotherapy in melanoma, Sci. Transl. Med 9 (2017) eaal4712. 10.1126/scitranslmed.aal4712. [DOI] [PubMed] [Google Scholar]
- [69].Mäkinen T, Jussila L, Veikkola T, Karpanen T, Kettunen MI, Pulkkanen KJ, Kauppinen R, Jackson DG, Kubo H, Nishikawa SI, Ylä-Herttuala S, Alitalo K, Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3, Nat. Med 7 (2001) 199–205. 10.1038/84651. [DOI] [PubMed] [Google Scholar]
- [70].Thomas SN, Rutkowski JM, Pasquier M, Kuan EL, Alitalo K, Randolph GJ, Swartz MA, Impaired Humoral Immunity and Tolerance in K14-VEGFR-3-Ig Mice That Lack Dermal Lymphatic Drainage, J. Immunol 189 (2012) 2181–2190. 10.4049/jimmunol.1103545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Wojciechowski S, Vihma M, Galbardi B, Anaïs V, Keuters MH, Salli A, Kari A, Koistinaho J, Noe FM, The CNS lymphatic system modulates the adaptive neuro-immune response in the perilesional cortex after brain trauma, BioRxiv. 7 (2019). 10.1101/821645. [DOI] [Google Scholar]
- [72].Oussoren C, Zuidema J, Crommelin DJA, Storm G, Lymphatic uptake and biodistribution of liposomes after subcutaneous injection. II. Influence of liposomal size, lipid composition and lipid dose, Biochim. Biophys. Acta -Biomembr 1328 (1997) 261–272. 10.1016/S0005-2736(97)00122-3. [DOI] [PubMed] [Google Scholar]
- [73].Deep Kaur C, Nahar M, Jain NK, Lymphatic targeting of zidovudine using surface-engineered liposomes, J. Drug Target 16 (2008). [DOI] [PubMed] [Google Scholar]
- [74].Kaminskas LM, Porter CJH, Targeting the lymphatics using dendritic polymers (dendrimers), Adv. Drug Deliv. Rev 63 (2011) 890–900. 10.1016/j.addr.2011.05.016. [DOI] [PubMed] [Google Scholar]
- [75].Francis DM, Manspeaker MP, Schudel A, Sestito LF, O’Melia MJ, Kissick HT, Pollack BP, Waller EK, Thomas SN, Blockade of immune checkpoints in lymph nodes through locoregional delivery augments cancer immunotherapy, Sci. Transl. Med 12 (2020) 1–12. 10.1126/scitranslmed.aay3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Thurber GM, Schmidt MM, Wittrup KD, Antibody tumor penetration: Transport opposed by systemic and antigen-mediated clearance, Adv. Drug Deliv. Rev 60 (2008) 1421–1434. 10.1016/j.addr.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Cai S, Xie Y, Davies NM, Cohen MS, Forrest ML, Pharmacokinetics and disposition of a localized lymphatic polymeric hyaluronan conjugate of cisplatin in rodents, J. Pharm. Sci 99 (2010) 2664–2671. 10.1002/jps.22016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Edwards GA, Porter CJH, Caliph SM, Khoo SM, Charman WN, Animal models for the study of intestinal lymphatic drug transport, Adv. Drug Deliv. Rev 50 (2001) 45–60. 10.1016/S0169-409X(01)00148-X. [DOI] [PubMed] [Google Scholar]
- [79].Hu L, Quach T, Han S, Lim SF, Yadav P, Senyschyn D, Trevaskis NL, Simpson JS, Porter CJH, Glyceride-Mimetic Prodrugs Incorporating Self-Immolative Spacers Promote Lymphatic Transport, Avoid First-Pass Metabolism, and Enhance Oral Bioavailability, Angew. Chem. Int. Ed 55 (2016) 13700–13705. 10.1002/anie.201604207. [DOI] [PubMed] [Google Scholar]
- [80].Zara GP, Bargoni A, Cavalli R, Fundaro A, Vighetto D, Gasco MR, Pharmacokinetics and tissue distribution of idarubicin-loaded solid lipid nanoparticles after duodenal administration to rats, J. Pharm. Sci 91 (2002). [DOI] [PubMed] [Google Scholar]
- [81].Harivardhan Reddy L, Sharma RK, Chuttani K, Mishra AK, Murthy RSR, Influence of administration route on tumor uptake and biodistribution of etoposide loaded solid lipid nanoparticles in Dalton’s lymphoma tumor bearing mice, J. Controlled Release 105 (2005) 185–198. 10.1016/j.jconrel.2005.02.028. [DOI] [PubMed] [Google Scholar]
- [82].Videira MA, Botelho MF, Santos AC, Gouveia LF, Pedroso De Lima JJ, Almeida AJ, Lymphatic uptake of pulmonary delivered radiolabelled solid lipid nanoparticles, J. Drug Target. 10 (2002) 607–613. 10.1080/1061186021000054933. [DOI] [PubMed] [Google Scholar]
- [83].Videira M, Almeida AJ, Fabra Angels, Preclinical evaluation of a pulmonary delivered paclitaxel-loaded lipid nanocarrier antitumor effect, Nanomedicine Nanotechnol. Biol. Med 8 (2012) 1208–1215. 10.1016/j.nano.2011.12.007. [DOI] [PubMed] [Google Scholar]
- [84].Francis DM, Thomas SN, Progress and opportunities for enhancing the delivery and efficacy of checkpoint inhibitors for cancer immunotherapy, Adv. Drug Deliv. Rev 114 (2017) 33–42. 10.1016/j.addr.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Kourtis IC, Hirosue S, de Titta A, Kontos S, Stegmann T, Hubbell JA, Swartz MA, Peripherally Administered Nanoparticles Target Monocytic Myeloid Cells, Secondary Lymphoid Organs and Tumors in Mice, PLoS ONE. 8 (2013). 10.1371/journal.pone.0061646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Broggi MAS, Maillat L, Clement CC, Bordry N, Corthésy P, Auger A, Matter M, Hamelin R, Potin L, Demurtas D, Romano E, Harari A, Speiser DE, Santambrogio L, Swartz MA, Tumor-associated factors are enriched in lymphatic exudate compared to plasma in metastatic melanoma patients, J. Exp. Med 216 (2019) 1091–1107. 10.1084/jem.20181618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Hood JL, San Roman S, Wickline SA, Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis, Cancer Res. 71 (2011) 3792–3801. 10.1158/0008-5472.CAN-10-4455. [DOI] [PubMed] [Google Scholar]
- [88].Clement CC, Santambrogio L, The lymph self-antigen repertoire, Front. Immunol 4 (2013) 1–5. 10.3389/fimmu.2013.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Sarkar K, Xue Y, Sant S, Host Response to Synthetic Versus Natural Biomaterials, in: Corradetti B (Ed.), Immune Response Implant. Mater. Devices, Springer International Publishing, Cham, 2017: pp. 81–105. 10.1007/978-3-319-45433-7_5. [DOI] [Google Scholar]
- [90].Campbell KT, Silva EA, Biomaterial Based Strategies for Engineering New Lymphatic Vasculature, Adv. Healthc. Mater 9 (2020). 10.1002/adhm.202000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Chang C, Seibel AJ, Song JW, Application of microscale culture technologies for studying lymphatic vessel biology, Microcirculation. 26 (2019). 10.1111/micc.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Dixon JB, Lymphatic lipid transport: sewer or subway?, Trends Endocrinol. Metab 21 (2010) 480–487. 10.1016/j.tem.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


