Figure 4. Multiple factors must be considered when designing therapeutics for immunomodulation via the lymphatic systems.
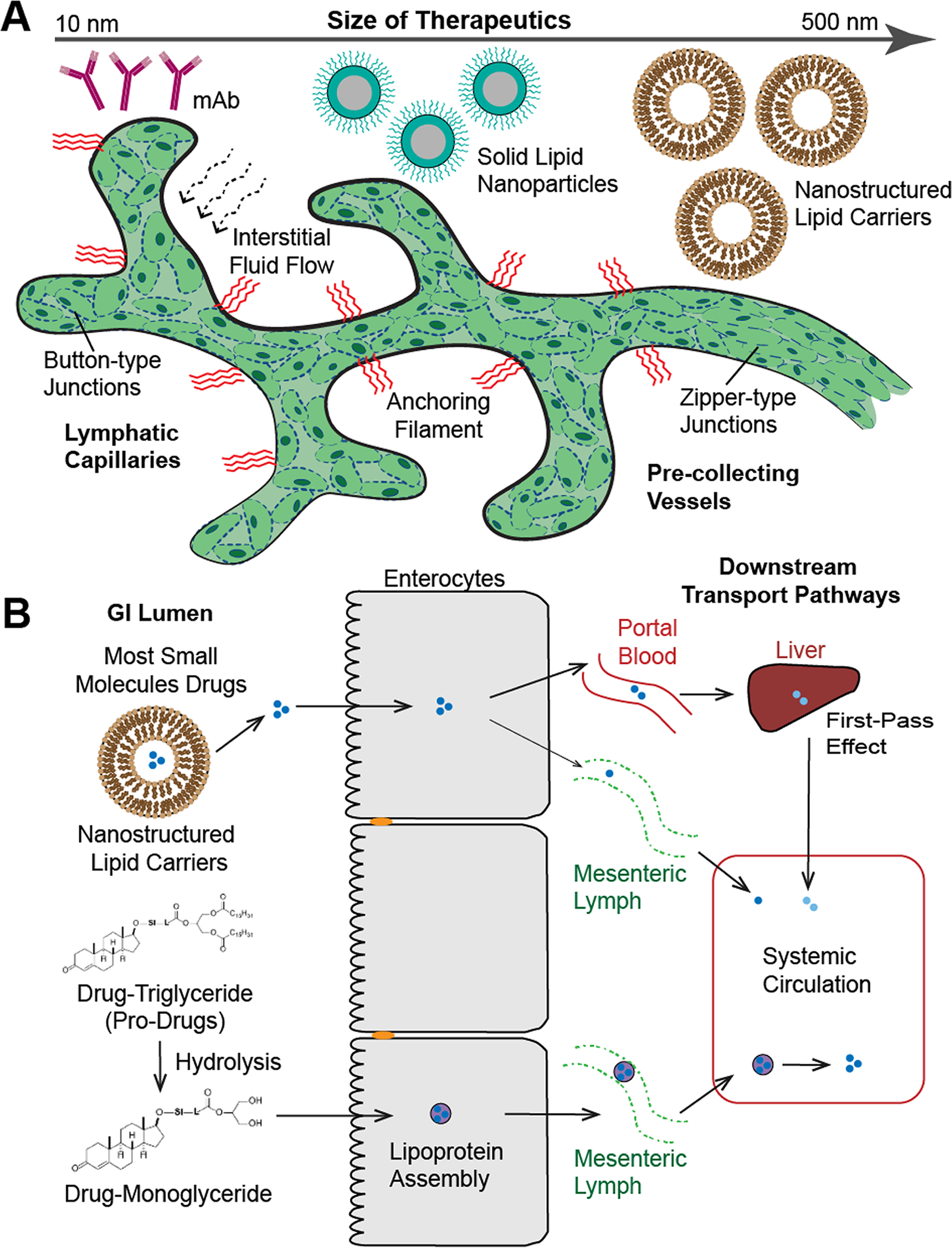
(A) Different types and sizes of therapeutics, such as monoclonal antibodies (mAb, 10 nm), solid lipid nanoparticles (200 nm), and nanostructured lipid carries (500 nm) that can be used to target the lymphatic system. As a reference, chylomicrons up to 1,000nm in diameter can be transported across the lacteals [92]. The specific structure of the lymphatic vessels being targeted must be considered, as their structure is organ-specific and vary in permeability depending on the types of junctions (button-type and zipper-type junctions), which will influence the possible biomaterials used for therapeutic delivery. (B) Schematic diagram of the different mechanisms of transport pathways following oral drug or pro-drug administration. The intestinal transport of lipid-based formulations (nanostructured lipid carries) through blood (major) and lymphatic circulation (minor). Pro-drug formulation as a drug-triglyceride undergoes hydrolysis into drug-monoglyceride, which will be further assembled into lipoprotein to enter the mesenteric lymph. Therefore, the drug can enter the systemic circulation by avoiding the first pass metabolism in the liver. Illustration in panel B was adapted with permission from [79].
