Abstract
Background
Gliomas are the most common primary brain tumor in adults. Current treatments involve surgery, radiation, and temozolomide (TMZ) chemotherapy; however, prognosis remains poor and new approaches are required. Circadian medicine aims to maximize treatment efficacy and/or minimize toxicity by timed delivery of medications in accordance with the daily rhythms of the patient. We published a retrospective study showing greater anti-tumor efficacy for the morning, relative to the evening, administration of TMZ in patients with glioblastoma. We conducted this prospective randomized trial to determine the feasibility, and potential clinical impact, of TMZ chronotherapy in patients with gliomas (NCT02781792).
Methods
Adult patients with gliomas (WHO grade II-IV) were enrolled prior to initiation of monthly TMZ therapy and were randomized to receive TMZ either in the morning (AM) before 10 am or in the evening (PM) after 8 pm. Pill diaries were recorded to measure compliance and FACT-Br quality of life (QoL) surveys were completed throughout treatment. Study compliance, adverse events (AE), and overall survival were compared between the two arms.
Results
A total of 35 evaluable patients, including 21 with GBM, were analyzed (18 AM patients and 17 PM patients). Compliance data demonstrated the feasibility of timed TMZ dosing. There were no significant differences in AEs, QoL, or survival between the arms.
Conclusions
Chronotherapy with TMZ is feasible. A larger study is needed to validate the effect of chronotherapy on clinical efficacy.
Keywords: chronotherapy, feasibility, gliomas, quality of life
Gliomas are the most common central nervous system (CNS) tumors.1 Glioblastoma (GBM) is the most aggressive glioma in adults with dismal survival between 12 and 20 months.1–3 Treatment for high-grade gliomas includes surgery, radiation with concomitant chemotherapy, and adjuvant temozolomide (TMZ) chemotherapy. TMZ has been shown to improve survival by 2.5 months2 and Tumor Treating Fields have improved survival by 5 months3; however, malignant gliomas continue to have a poor prognosis. In this study, we investigate timed dosing of TMZ for optimization of the current treatment regimen, which is imperative for improving survival.
Timing drug delivery to align with the biological rhythms of the patient is known as circadian medicine or chronotherapy. Several cancers have benefitted from a circadian medicine approach, through reduction of side effects or enhanced efficacy of the drug at certain times of day. Patients with pediatric acute lymphoblastic leukemia benefited from evening treatment with 6-mercaptopurine and methotrexate.4 Patients with advanced ovarian cancer saw reduced side effects from evening cisplatin and morning doxorubicin.5 Male patients with metastatic colorectal cancer benefited from chronomodulated 5-fluorouracil and leucovorin infused during the night and oxaliplatin during the day, while female patients did better with non-chronomodulated therapy.6 TMZ is an oral DNA alkylating agent that readily crosses the blood-brain barrier7 with a short half-life of 2 hours,8 making it an ideal chronotherapy candidate.
Our recent retrospective study revealed a 3.6-month longer survival in GBM patients who took TMZ in the morning compared to evening and a 6-month extension of survival in patients with O6-methylguanine-DNA methyltransferase (MGMT) promoter methylated tumors.9 Studies in cell lines shed some light on the potential mechanisms by which circadian medicine can exert its effects in human trials. A cellular model of GBM showed a time-of-day-dependent sensitivity to TMZ treatment, with elevated DNA damage and 3-fold increased cell death corresponding to treatment at the peak of core circadian clock gene, Bmal1.10 This time-of-day effect in vitro may be due to daily rhythms in metabolism,11 DNA repair processes,12 and cell cycle regulation.13 These findings support the hypothesis that morning dosing would be associated with better outcome in a large prospective clinical trial.
Based on the positive impact of chronomedicine in other cancer types and in our published retrospective GBM study, the preclinical evidence supporting daily rhythms in GBM sensitivity to TMZ, and the significant unmet need of optimized treatment in glioma, we conducted a randomized phase II trial to evaluate the feasibility of administering TMZ to glioma patients in the morning or evening. We collected safety and feasibility data, and although not powered for efficacy, summarized preliminary findings of survival analysis. This study is registered on clinicaltrials.gov (NCT02781792).
Methods
Patients
Eligible patients were >18 years of age with a diagnosis of either newly diagnosed or recurrent high-grade glioma (WHO grade III and IV) or high-risk WHO grade II gliomas (IDH wild-type, TERT promoter mutation, EGFR amplification, or PTEN deletion), planning to begin treatment with monthly adjuvant high-dose TMZ therapy, Karnofsky performance status (KPS) >60%, and being able to understand and willingly sign an institutional review board (IRB)-approved written informed consent document. This study was available to both women and minorities as long as they met the above eligibility criteria. Pregnant and/or breastfeeding patients were excluded.
Study Procedures
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and were approved by the Washington University in St. Louis IRB.
All procedures were conducted in accordance with the 1964 Declaration of Helsinki and its amendments. Patients were recruited from the Washington University Siteman Cancer Center in Saint Louis, Missouri, from June 2016 to August 2020. Participants were approached for the study during their scheduled clinic visits. All participants signed informed consent.
Eligible patients were randomized in a 1:1 ratio to either the AM arm or PM arm, which required participants to either take TMZ before 10 AM or after 8 PM. The randomization was performed by the clinical trial team through the university’s Online Collaborative Research Environment (OnCore), a comprehensive, web-based, vendor-supported clinical trial management system (CTMS).
Intervention
TMZ was given to eligible patients with a dosing of 150-200 mg/m2 from days 1 to 5 of a 28-day treatment cycle. Patients were instructed to maintain a medication diary, where they would record the date, time, and a number of tablets taken on each day. Patients who were unable to take their TMZ dose at the assigned time were encouraged to take it as soon as possible, rather than not taking it at all as long as the next scheduled dose was at least 12 hours away. The clinical research coordinator called patients the day before their scheduled visits to verify diary completion and remind them to bring their pill diaries along for their visits.
Assessments and Endpoints
The primary endpoint of this randomized trial was feasibility of patient compliance to TMZ chronotherapy, defined as taking TMZ at the assigned time at least 80% of the time.
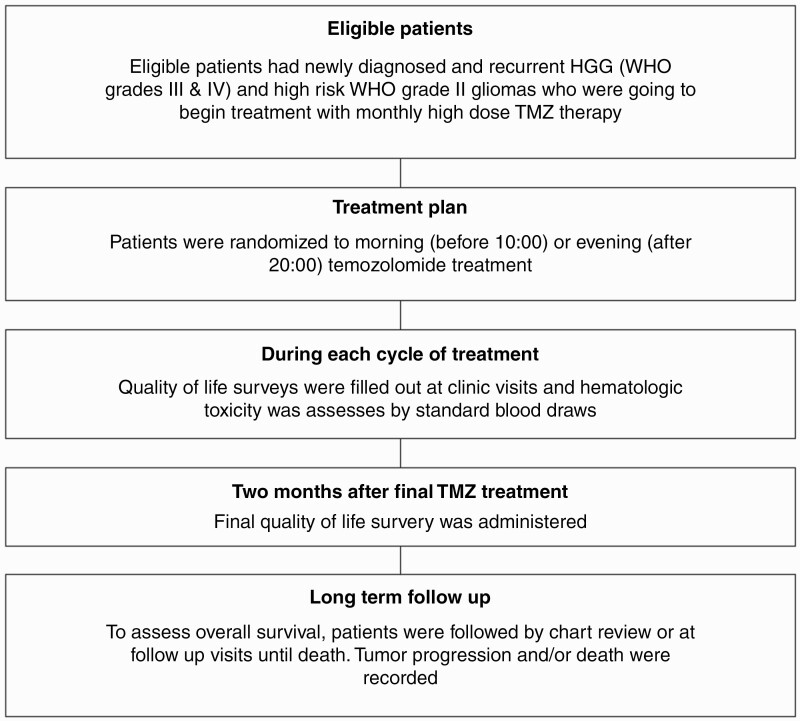
Patients were evaluated with monthly laboratory assessments, including complete blood counts (CBCs) and comprehensive metabolic panels (CMPs) at each clinic visit (Figure 1). The Functional Assessment of Cancer Therapy—Brain (FACT-Br) was completed during 10-15 minutes of each monthly visit. FACT-Br subscale score and total score were calculated per FACIT guidelines. A higher score indicates better quality of life (QoL).14 A study log that recorded the highest grade for each hematologic toxicity for every patient was also compiled. Patients were removed from the study if they had any grade 4 or 5 toxicity, progression of the disease, or death. Finally, patients who completed treatment or stopped treatment (due to either progression, adverse events [AEs], or withdrawal from treatment or follow-up) were followed by chart review after administration of their final QoL survey.
Figure 1.
Outline of eligibility, treatment, assessment, and follow-up of patients enrolled in the trial.
Statistical Analysis
This feasibility study was designed to include at least 30 patients, according to the reference15 which recommended a sample size of 10-20 patients for reasonable precision in estimating preliminary information based on extensive simulations for plot translational studies. Patient characteristics and AE incidence were compared using a two-sample t test or Fisher’s exact test, as appropriate. FACT-Br start-end differences in the score were compared between groups using a two-sample t test. Two-way ANOVAs were applied to both monthly FACT-Br and cell count data with the factors of time, treatment arm, and the time-arm interaction to compare time difference within a group and group difference at each time. All analyses were performed using GraphPad Prism (Version 9.2.0).
Results
Patient Characteristics
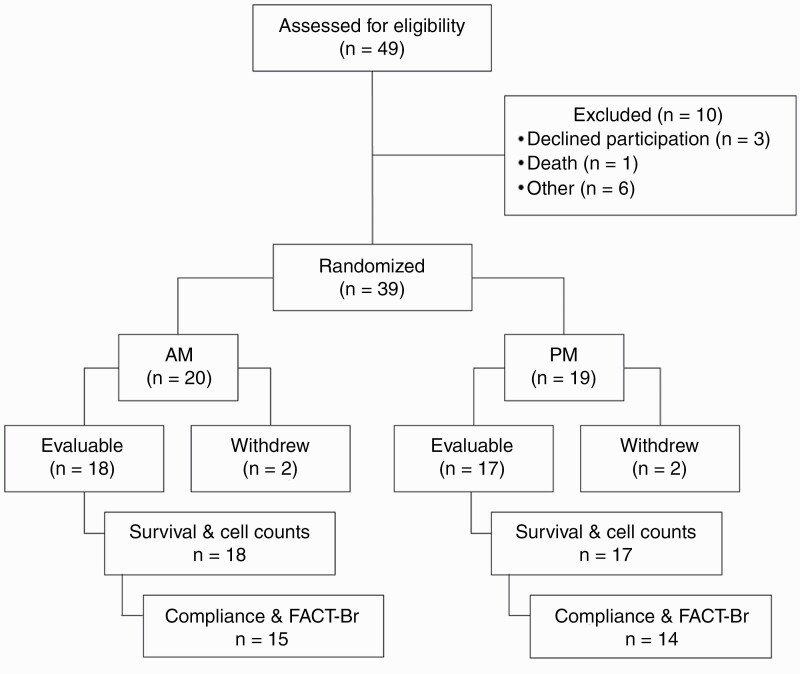
A total of 49 glioma patients were consented to the study between June 2016 and August 2020. On screening, 10 of these were deemed ineligible to participate based on the key study inclusion and exclusion criteria (Figure 2). Inclusion criteria included a diagnosis of WHO grade III and IV or high-risk WHO grade II glioma, a treatment plan employing monthly high-dose TMZ, a KPS ≥60%, ability to consent, and at least 18 years of age. Among the 39 patients eligible for treatment on the study, four later withdrew from the study, leaving 35 patients (18 AM patients and 17 PM patients) evaluable for survival and cell counts. To be evaluable, patients must have completed at least one cycle (5 days) of TMZ therapy. Mean age was 56 at the time of diagnosis (Table 1). The majority of patients (60%) had a diagnosis of WHO grade IV glioblastoma multiforme and 69% of patients had a KPS >90.
Figure 2.
Thirty-nine patients of 49 assessed for eligibility were randomized into study arms. All 39 patients were included in the analysis of adverse events. Of these evaluable patients, all received at least one cycle of TMZ and were included in survival and blood laboratory test analyses. Twenty-nine patients receiving ≥2 cycles of temozolomide were included in compliance and FACT-Br analyses. Abbreviations: FACT-Br, Functional Assessment of Cancer Therapy—Brain; TMZ, temozolomide.
Table 1.
Summary of Patient Baseline Characteristics
| AM Dosing (n = 18) | PM Dosing (n = 17) | All Patients (n = 35) | P-value | |
|---|---|---|---|---|
| Demographics | ||||
| Mean age at diagnosis (range) | 59.72 (37-75) | 52.71 (20-81) | 56.31 (20-81) | .20a |
| Male (%) | 8 (44) | 12 (70) | 20 (57) | .18b |
| Race (%) | ||||
| White, non-Hispanic | 16 (89) | 16 (94) | 32 (91) | .23c |
| Black/African American, non-Hispanic | 2 (11) | 0 (0) | 2 (6) | |
| Asian or Pacific Highlander | 0 (0) | 1 (6) | 1 (3) | |
| Disease characteristics | ||||
| Histology | ||||
| Anaplastic astrocytoma: No. (%) | 2 (11) | 2 (12) | 4 (11) | .50c |
| Diffuse astrocytoma: No. (%) | 2 (11) | 3 (18) | 5 (14) | |
| Anaplastic oligodendroglioma: No. (%) | 1 (6) | 1 (6) | 2 (6) | |
| Glioblastoma: No. (%) | 10 (55) | 11 (64) | 20 (57) | |
| Oligodendroglioma: No. (%) | 3 (17) | 0 (0) | 3 (9) | |
| WHO grade | ||||
| II: No. (%) | 4 (22) | 3 (17) | 7 (20) | .86c |
| III: No. (%) | 4(22) | 3 (17) | 7 (20) | |
| IV: No. (%) | 10 (56) | 11 (65) | 21 (60) | |
| KPS | ||||
| 60-80: No. (%) | 6 (34) | 5 (30) | 11 (31) | .99c |
| 90-100: No. (%) | 12 (66) | 12 (70) | 24 (69) | |
| MGMT promoter methylation status | ||||
| Methylated: No. (%) | 6 (33.33) | 3 (18) | 9 (26) | .44c |
| Unmethylated: No. (%) | 6 (33.33) | 9 (53) | 15 (43) | |
| Unknown: No. (%) | 6 (33.33) | 5 (29) | 11 (31) | |
| IDH mutation status | ||||
| Wild type | 11 (69) | 12 (70) | 23 (66) | .40c |
| Mutated | 7 (31) | 4 (24) | 11 (31) | |
| Unknown | 0 (0) | 1 (6) | 1 (3) |
Abbreviations: IDH, isocitrate dehydrogenase; KPS, Karnofsky performance status; MGMT, O6-methylguanine-DNA methyltransferase.
aWelch’s test.
bFisher’s exact test.
cChi-square test.
Feasibility
To assess the feasibility, we analyzed 29 patients (AM n = 15, PM n = 14) who successfully completed a medication diary documenting the time that they took their medication in each cycle. In both arms, the median number of treatment cycles was 6 cycles, ranging from 2 to 12. The AM arm included 108 person-time TMZ administration and the PM included 94 person-time TMZ administration. Patients were 100% and 96.8% compliant, taking TMZ at the prescribed time either before 10 am or after 8 pm, respectively. Of the three noncompliant administrations in the PM group, two involved taking the drug outside the prescribed time window and in the third, the patient could not recall when the drug was taken.
Adverse Events
Per the study AEs log, we recorded the worst grade clinical and hematological treatment-emergent AE (TEAE) by system organ class for each patient (Table 2). The PM group reported more grade 1 AEs, both non-hematological and hematological. There were 4 patients reporting grade 3 or higher TEAEs in the AM arm and 1 in the PM arm. For non-hematological TEAEs, 1 AM patient reported grade 3 nausea and 1 PM patient reported grade 3 generalized muscle weakness. For hematological TEAEs, 3 AM patients reported grade 3 or higher hematological AEs, compared to none in the PM arm, demonstrating a higher incidence for higher grade AEs in the AM arm compared to the PM arm, albeit not statistically significant (P = .34).
Table 2.
Clinical and Hematological Adverse Events Related to Temozolomide (TMZ) Treatment
| AM Group (N = 20) % Grade 3-10 (n = 2) % Grade 4-10 (n = 2) |
PM Group (N = 19) % Grade 3-5.3 (n = 1) % Grade 4-0 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Clinical adverse events | ||||||||
| Constipation | 5 (25%) | 5 (26.3%) | ||||||
| Nausea | 5 (25%) | 1 (5%) | 9 (47.4%) | |||||
| Vomiting | 3 (15%) | 7 (36.8%) | ||||||
| Fatigue | 2 (10%) | 2 (10.53%) | 3 (15.8%) | |||||
| Rash | 1 (5%) | 1 (5.3%) | ||||||
| Diarrhea | 1 (5%) | |||||||
| Anorexia | 3 (15%) | 3 (15.8%) | 1 (5.3%) | |||||
| Generalized muscle weakness | 2 (10%) | 1 (5.3%) | 2 (10.53%) | 1 (5.3%) | ||||
| Dysphasia | ||||||||
| Diarrhea | 1 (5.3%) | 1 (5.3%) | ||||||
| Laboratory | ||||||||
| Platelet count decreased | 1 (5%) | 1 (5%) | 2 (10.53%) | 2 (10.53%) | ||||
| Lymphocyte count decreased | 1 (5%) | 2 (10.53%) | 1 (5.3%) | |||||
| Anemia | 3 (15%) | 4 (21.1%) | ||||||
| Neutrophil count decreased | 1 (5.3%) | |||||||
| Lymphocyte count increased | 1 (5%) | 1 (5%) | 4 (21.1%) | 1 (5.3%) | ||||
| Aspartate aminotransferase increased | 1 (5%) | |||||||
| Bilirubin increased | 1 (5%) | 1 (5%) | ||||||
| Alkaline phosphatase | 1 (5%) | |||||||
| White blood cells decreased | 4 (20%) | 2 (10%) | 1 (5.3%) | |||||
| Alanine aminotransferase increased | 1 (5%) | 1 (5.3%) |
Blood laboratory test data were evaluated across all time points for the 35 patients who completed at least one cycle of TMZ. Specifically, white blood cells (WBC), lymphocytes, hemoglobin (Hgb), and platelets (Plt) (Supplementary Figure 1A–D) were compared between the two arms over a 6-month period. For all cell counts, there was no difference between AM and PM patients during the first 6 months of treatment, regardless of disease stage. Platelet counts were significantly higher in lower grade (grade II-III) patients at months 2, 5, and 6 of treatment.
Quality of Life
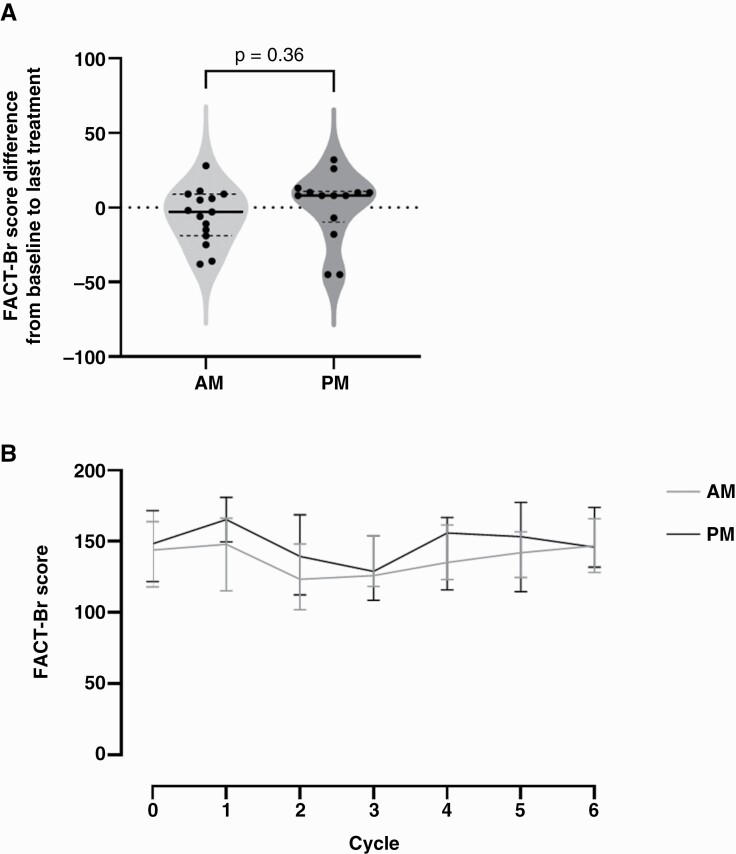
The FACT-Br QoL survey, used extensively in the field to monitor QoL of brain cancer patients,16 was administered to the patients during each monthly clinic visit. The FACT-Br is composed of five subscales: physical well-being (PWB), social well-being (SWB), emotional well-being (EWB), functional well-being (FWB), and the brain cancer subscale (Br). There was no difference in FACT-Br scores between AM and PM groups at the start of treatment or at any point throughout treatment. There was also no difference in FACT-Br scores within each group, between the start and end of treatment (Figure 3).
Figure 3.
Quality of life was assessed using the FACT-Br composite score for 29 patients completing at least 2 cycles of treatment (15 AM, 14 PM). (A) Start and end scores were similar for patients in the morning and evening groups (t-test, P = .36). (B) We found FACT-Br scores (presented as median with IQR intervals) did not differ (P = .69, 2-way ANOVA) over 6 cycles of TMZ treatment between patients treated in the morning (AM, gray) and in the evening (PM, black). Abbreviations: ANOVA, analysis of variance; FACT-Br, Functional Assessment of Cancer Therapy—Brain; IQR, interquartile range; TMZ, temozolomide.
Patient Survival
Among newly diagnosed IDH wild-type patients, median overall survival was similar between AM and PM treated arms in both GBM and grade II-III gliomas (Supplementary Figure 2). Similar survival outcomes were also observed in recurrent patients. Subgroup analyses showed that IDH mutation and MGMT promoter methylation also extend overall survival, as previously published.17,18
Discussion
In cancer, circadian medicine has been shown to affect survival or tumor cell death in diverse cancers including metastatic colorectal cancer (5-fluorouracil and leucovorin in early morning, oxaliplatin in afternoon)6 (reviewed in Lévi19), acute lymphoblastic leukemia (evening),4 and some ovarian and genitourinary cancers (doxorubicin in the morning, cisplatin in the evening, fluorodeoxyuridine in the evening).20 Recent papers have highlighted strategies for identifying drugs that target circadian products and predicting times of optimal dosing.21–25 Yet, chronotherapy has not been widely tested or adopted for most cancers in part because of the complexity of adding time of treatment to clinical trials. In this study, we determined that timed administration of adjuvant TMZ chemotherapy is feasible in glioma patients. The 98% compliance observed in this trial improves upon average compliance observed with Tumor Treating Fields use (80%-90%26). Given the feasibility of timed dosing, TMZ chronotherapy may offer advantages in gliomas for which few effective treatment options exist.
We found that AEs were consistent with those previously reported and did not significantly differ between arms, although the AM group recorded 3 grade 3/4 hematological AEs while the PM group recorded no hematological AEs. AEs related to TMZ therapy most commonly consist of nausea, vomiting, constipation, and myelosuppression.27 These AEs can result in dose reductions and in some cases—drug discontinuation; however, the time of dosing has not been specified in previous studies of TMZ therapy. In colorectal cancer,6,28 timed peak dosing of therapy has been applied to reduce the severity of side effects, enabling patients to adhere longer to drug treatment regimen and avoid dose reductions. However, these results indicate that TMZ delivery times could be selected primarily for tumor suppression.
Previous work done in vitro and in a retrospective chart study supported greater efficacy of TMZ when dosed at the daily peak of the clock gene, Bmal1, in cells10 or in the morning in humans.9 Postmortem human cortical samples show Bmal1 transcription peaking about 6 h after dusk (ZT18).29 This suggests that TMZ treatments in the morning may improve outcomes by targeting gliomas at a time when they are more likely to fail to repair the double-stranded breaks in DNA induced by TMZ. Due to the small sample size and heterogeneous patient population in this study, a larger study is needed to assess the survival benefit of morning TMZ.
In addition to daily timed dosing, there are preclinical studies targeting the circadian clock pharmacologically in glioma stem cells. Small molecules, SR9011 and SR9009, agonists of REV-ERBα/β which inhibit Bmal1 transcription,30 and KL001 and its derivative SHP656, CRY1/2 stabilizers which prevent their repression of the BMAL1 and CLOCK heterodimer binding to the promoter regions of Per1/2 and Cry1/2,31 reduced glioma stem cell proliferation independently, and to a greater extent when combined in vitro. In vivo, both SR9009 and SHP656 reduce tumor burden and extend mouse survival.30,31 In mouse models of glioma, these pharmacological agents worked equally as well as TMZ. Their efficacy has not yet been tested as a function of time of day.
While the results of this study are exciting, they are also preliminary and limited in their generalizability. The limitations of this study include its small study population, single-institute design, delays in enrollment related to COVID-19, and the lack of information about the patients’ sleep-wake schedules.
Chronomedicine has been applied successfully in oncology to decrease tumor burden and reduce drug side effects in a handful of cancer types; however, the potential for chronomedicine to improve patient outcomes has not been investigated widely or in large multi-site studies. A larger, multi-institute study is needed to determine if morning TMZ dosing confers a significant survival benefit to affect the standard of treatment for glioma patients. This larger study could also assess patient chronotype to correlate it with TMZ timing and outcome measurements. For example, it may be that morning TMZ offers greater benefits for patients that prefer to wake in a specific time window or fails to improve outcomes for patients with disrupted daily sleep-wake rhythms. Future studies should correlate chemotherapy outcomes with circadian biomarkers such as the amplitude and time of daily peak wrist actigraphy, body temperature, melatonin, or cortisol. The findings presented here support a large, multi-site clinical trial to assess the efficacy of chronotherapy in glioma.
Supplementary Material
Funding
This work was supported by the Research Fund from the Division of Oncology, Washington University in St. Louis (to J.L.C), the Alvin J. Siteman Cancer Center Siteman Investment Program through funding from The Foundation for Barnes-Jewish Hospital and The Barnard Trust (to J.B.R., J.L.C., and E.D.H.), the Children’s Discovery Institute (to J.B.R. and E.D.H.), the National Institutes of Health National Cancer Institute (F31CA250161 to A.R.D.), and the Siteman Cancer Center Early Phase Clinical Research Support (to J.L.C).
Conflict of interest statement. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or nonfinancial interest in the subject matter or materials discussed in this manuscript.
References
- 1. Wesseling P, Capper D. WHO 2016 classification of gliomas. Neuropathol Appl Neurobiol. 2018;44(2):139–150. [DOI] [PubMed] [Google Scholar]
- 2. Stupp R, Mason WP, van den Bent MJ, et al. . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 3. Stupp R, Taillibert S, Kanner A, et al. . Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318(23):2306–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rivard GE, Infante-Rivard C, Dresse M-F, Leclerc J-M, Champagne J. Circadian time-dependent response of childhood lymphoblastic leukemia to chemotherapy: a long-term follow-up study of survival. Chronobiol Int. 1993;10(3):201–204. [DOI] [PubMed] [Google Scholar]
- 5. Hrushesky WJ. Circadian timing of cancer chemotherapy. Science. 1985;228(4695):73–75. [DOI] [PubMed] [Google Scholar]
- 6. Giacchetti S, Bjarnason G, Garufi C, et al. . Phase III trial comparing 4-day chronomodulated therapy versus 2-day conventional delivery of fluorouracil, leucovorin, and oxaliplatin as first-line chemotherapy of metastatic colorectal cancer: the European Organisation for Research and Treatment of Cancer Chronotherapy Group. J Clin Oncol. 2006;24(22):3562–3569. [DOI] [PubMed] [Google Scholar]
- 7. Ballesta A, Zhou Q, Zhang X, Lv H, Gallo JM. Multiscale design of cell-type-specific pharmacokinetic/pharmacodynamic models for personalized medicine: application to temozolomide in brain tumors. CPT Pharmacomet Syst Pharmacol. 2014;3(4):e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ostermann S, Csajka C, Buclin T, et al. . Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin Cancer Res. 2004;10(11):3728–3736. [DOI] [PubMed] [Google Scholar]
- 9. Damato AR, Luo J, Katumba RGN, et al. . Temozolomide chronotherapy in patients with glioblastoma: a retrospective single institute study. Neurooncol Adv. 2021;3(1):vdab041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Slat EA, Sponagel J, Marpegan L, et al. . Cell-intrinsic, Bmal1-dependent circadian regulation of temozolomide sensitivity in glioblastoma. J Biol Rhythms. 2017;32(2):121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Masri S, Sassone-Corsi P. The emerging link between cancer, metabolism and circadian rhythms. Nat Med. 2018;24(12):1795–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang Y, Adebali O, Wu G, et al. . Cisplatin-DNA adduct repair of transcribed genes is controlled by two circadian programs in mouse tissues. Proc Natl Acad Sci USA. 2018;115(21):E4777–E4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Masri S, Cervantes M, Sassone-Corsi P. The circadian clock and cell cycle: interconnected biological circuits. Curr Opin Cell Biol. 2013;25(6):730–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holzner B, Kemmler G, Cella D, et al. . Normative data for functional assessment of cancer therapy—general scale and its use for the interpretation of quality of life scores in cancer survivors. Acta Oncol. 2004;43:153–160. [DOI] [PubMed] [Google Scholar]
- 15. Piantadosi S. Translational clinical trials: an entropy-based approach to sample size. Clin Trials. 2005;2(2):182–192. [DOI] [PubMed] [Google Scholar]
- 16. Lien K, Zeng L, Nguyen J, et al. . FACT-Br for assessment of quality of life in patients receiving treatment for brain metastases: a literature review. Expert Rev Pharmacoecon Outcomes Res. 2011;11(6):701–708. [DOI] [PubMed] [Google Scholar]
- 17. Houillier C, Wang X, Kaloshi G, et al. . IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low-grade gliomas. Neurology. 2010;75(17):1560–1566. [DOI] [PubMed] [Google Scholar]
- 18. Weller M, Stupp R, Reifenberger G, et al. . MGMT promoter methylation in malignant gliomas: ready for personalized medicine? Nat Rev Neurol. 2010;6(1):39–51. [DOI] [PubMed] [Google Scholar]
- 19. Lévi F. Circadian chronotherapy for human cancers. Lancet Oncol. 2001;2(5):307–315. [DOI] [PubMed] [Google Scholar]
- 20. Kobayashi M, Wood PA, Hrushesky WJM. Circadian chemotherapy for gynecological and genitourinary cancers. Chronobiol Int. 2002;19(1):237–251. [DOI] [PubMed] [Google Scholar]
- 21. Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci USA. 2014;111(45):16219–16224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sancar A, Gelder RNV. Clocks, cancer, and chronochemotherapy. Science. 2021;371(6524):42– 45. [DOI] [PubMed] [Google Scholar]
- 23. Ruben MD, Wu G, Smith DF, et al. . A database of tissue-specific rhythmically expressed human genes has potential applications in circadian medicine. Sci Transl Med. 2018;10(458):eaat8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anafi RC, Francey LJ, Hogenesch JB, Kim J. CYCLOPS reveals human transcriptional rhythms in health and disease. Proc Natl Acad Sci USA. 2017;114(20):5312–5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Selfridge JM, Gotoh T, Schiffhauer S, et al. . Chronotherapy: intuitive, sound, founded…but not broadly applied. Drugs. 2016;76(16):1507–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Toms SA, Kim CY, Nicholas G, Ram Z. Increased compliance with tumor treating fields therapy is prognostic for improved survival in the treatment of glioblastoma: a subgroup analysis of the EF-14 phase III trial. J Neurooncol. 2019;141(2):467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tan AC, Ashley DM, López GY, et al. . Management of glioblastoma: state of the art and future directions. CA Cancer J Clin. 2020;70(4):299–312. [DOI] [PubMed] [Google Scholar]
- 28. Lévi FA, Zidani R, Vannetzel JM, et al. . Chronomodulated versus fixed-infusion-rate delivery of ambulatory chemotherapy with oxaliplatin, fluorouracil, and folinic acid (leucovorin) in patients with colorectal cancer metastases: a randomized multi-institutional trial. J Natl Cancer Inst. 1994;86(21):1608–1617. [DOI] [PubMed] [Google Scholar]
- 29. Logan RW, Xue X, Ketchesin KD, et al. . Sex differences in molecular rhythms in the human cortex. Biol Psychiatry. 2021;91(1):152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sulli G, Rommel A, Wang X, et al. . Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence. Nature. 2018;553(7688):351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dong Z, Zhang G, Qu M, et al. . Targeting glioblastoma stem cells through disruption of the circadian clock. Cancer Discov. 2019;9(11):1556–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.