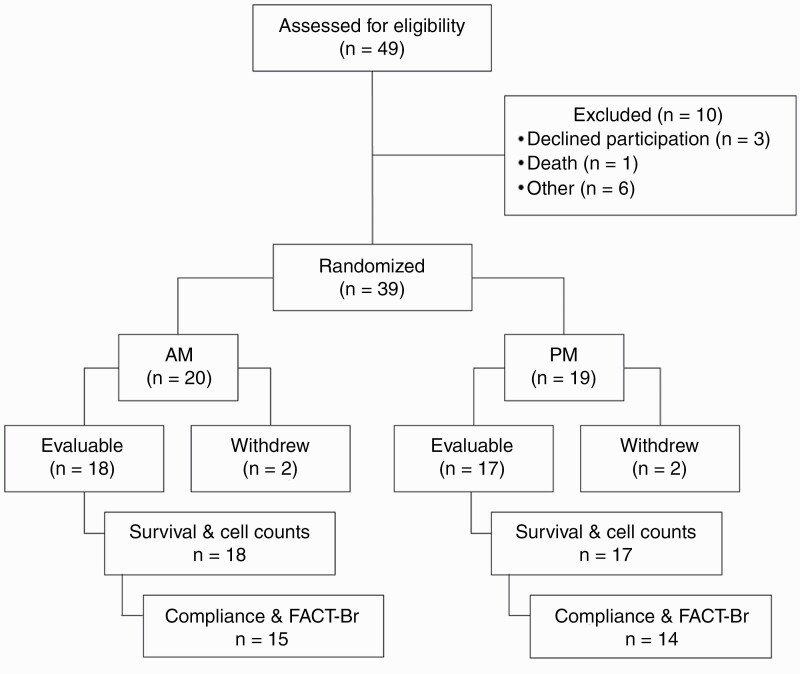
Figure 2.
Thirty-nine patients of 49 assessed for eligibility were randomized into study arms. All 39 patients were included in the analysis of adverse events. Of these evaluable patients, all received at least one cycle of TMZ and were included in survival and blood laboratory test analyses. Twenty-nine patients receiving ≥2 cycles of temozolomide were included in compliance and FACT-Br analyses. Abbreviations: FACT-Br, Functional Assessment of Cancer Therapy—Brain; TMZ, temozolomide.