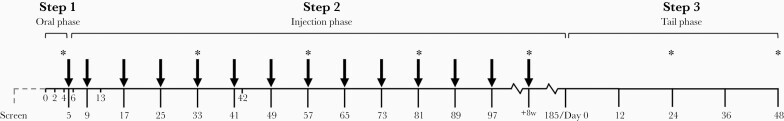
Figure 1.
HIV Prevention Trials Network (HPTN) 084 study schema. The study included 3 steps: an oral lead-in phase (step 1), an injection phase (step 2), and an open-label tail phase (step 3). In the tenofovir disoproxil fumarate (TDF)/emtricitabine (FTC) arm, participants received daily TDF/FTC in steps 1 and 2 (coformulated tablets; 300-mg TDF plus 200-mg FTC), along with placebo tablets (in step 1) and placebo injections (in step 2). In the cabotegravir (CAB) arm, participants received oral daily CAB in step 1 (30-mg tablets) and long-acting CAB (CAB-LA) injections in step 2 (600 mg; 3 mL), with placebo tablets in steps 1 and 2. All participants received daily open-label TDF/FTC in step 3. The first and second injections were given 4 weeks apart at weeks 5 and 9; participants then received injections every 8 weeks (arrows). Plasma samples were collected at every visit. Dried blood spot (DBS) samples were prepared and stored at weeks 4 and week 33, followed by every third injection visit (asterisks); DBS samples were also stored at weeks 24 and 48 in step 3. Study drugs were discontinued if participants had a reactive or positive human immunodeficiency virus (HIV) test result, declined drug administration, or had significant adverse events. Study drugs were also stopped for other reasons at the discretion of the site principal investigator.