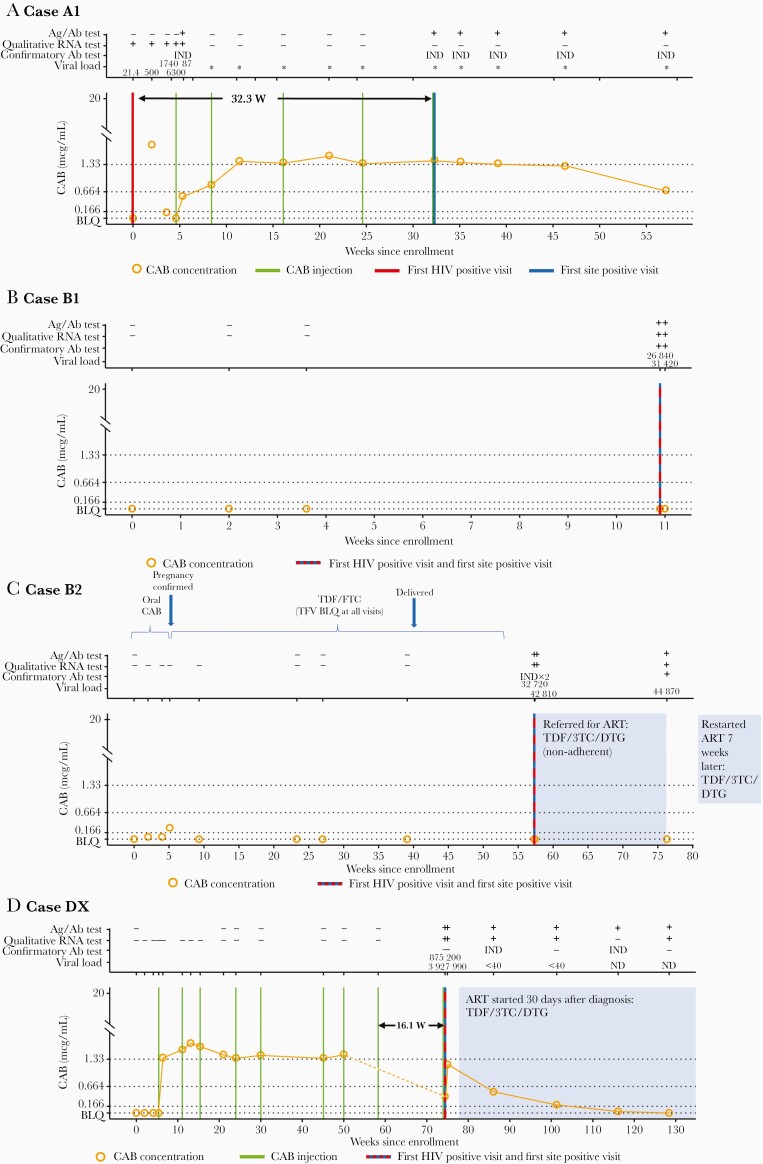
Figure 2.
Case summaries for the cabotegravir (CAB) arm), including laboratory results and key events for participants (cases A1, B1, B2, and DX). The full set of laboratory data for these cases, including results from testing performed at study sites and results from human immunodeficiency virus (HIV) resistance testing, is provided in Supplementary File 2. Annotations above each graph show results obtained from testing performed at the HIV Prevention Trials Network (HPTN) Laboratory Center, displayed as reactive/positive (plus sign [+]), negative/nonreactive (minus sign [−]), or indeterminate (IND). Viral load values represent HIV RNA copies per milliliter; a value <40 copies/mL indicates that HIV RNA was detected but was below the limit of quantification (BLQ). Asterisks signify negative single-copy RNA test results (no RNA detected). The graphs show plasma CAB concentrations and key events by weeks since study enrollment. The arrow in A shows the weeks between the first HIV-positive visit and the first site-positive visit (visit at which the site had a reactive or positive HIV test result); the arrow in D, the weeks between the eighth injection and the first HIV-positive visit. Horizontal lines indicate the following CAB concentration cutoffs: 1.33 mcg/mL, 8× the protein-adjusted in vitro 90% inhibitory concentration (PA-IC90); 0.664 mcg/mL, 4× PA-IC90, and 0.166 mcg/mL, 1× PA-IC90. BLQ indicates that the CAB concentration was <0.025 mcg/mL, shaded areas indicate periods of antiretroviral treatment (ART). Abbreviations: 3TC: lamivudine; Ab, antibody; Ag/Ab, antigen-antibody test; DTG, dolutegravir; FTC, emtricitabine. ND, not detected; TDF, tenofovir disoproxil fumarate; TFV, tenofovir.