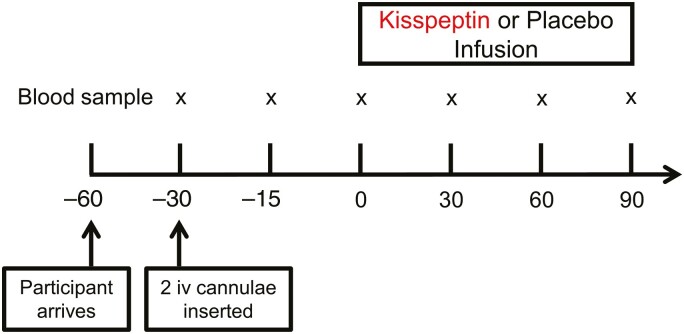
Figure 1.
Experimental protocol for human in vivo clinical study. Twenty-six healthy men participated in a randomized, double-blind, 2-way crossover, placebo-controlled study. Each participant attended 2 study visits; 1 for intravenous (iv) administration of kisspeptin (1 nmol/kg/hour) and 1 for intravenous administration of rate-matched placebo (vehicle), in random order. Blood samples were taken every 30 minutes (x). Participants remained fasted and supine throughout. n = 26 healthy eugonadal men.