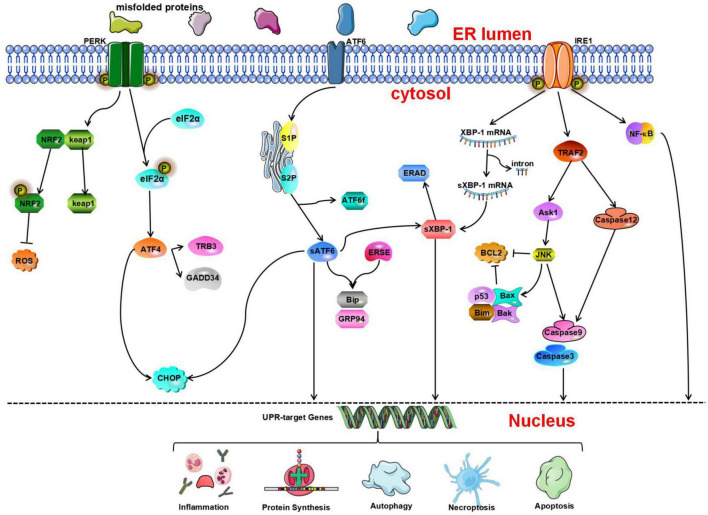
FIGURE 1.
The unfolded protein response (UPR) determines cell fate through the protein kinase R-like endoplasmic reticulum kinase (PERK), inositol-requiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF 6) pathways. Nuclear factor erythroid 2-related factor 2 (NRF2) is phosphorylated by PERK and dissociates from Kelch-like ECH-associated protein 1 (Keap1) under oxidative stress conditions and then activates the expression of NRF2-dependent antioxidant genes. p-eIF2a can inhibit protein synthesis. Activated ATF4 induces the expression of growth arrest and DNA damage-inducible gene 34 (GADD34) and tribbles-related protein 3 (TRB3). ATF6 is cleaved by serene protease site 1 protease and site 2 protease (S1P and S2P, respectively) to generate ATF6f and activated sATF6. Then, it combines with endoplasmic reticulum stress response elements (ERSEs) to regulate and activate the expression of BiP and glucose regulating protein 94 (GRP94). In addition, IRE1 contributes to ERS-mediated apoptosis through the tumor necrosis factor receptor-associated factor 2- activate apoptosis signal-regulating kinase-1-c-Jun N-terminal kinase (TRAF2-ASK1-JNK) and caspase-12 pathways. In addition, inositol-requiring enzyme 1α (IRE1α) can activate the nuclear factor κB (NF-κB) signaling pathway to initiate inflammatory reactions.