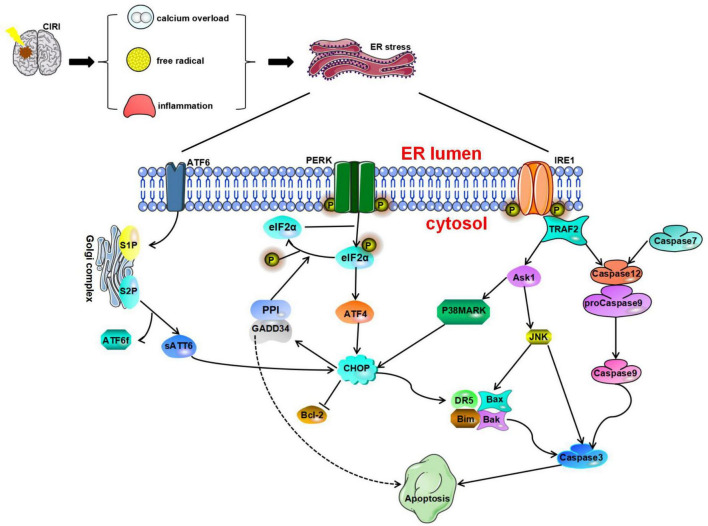
FIGURE 2.
Endoplasmic reticulum stress is a harmful process that induces apoptosis mediated by CAAT/enhancer-binding protein (C/EBP) homologous protein (CHOP), caspase-12, and JNK. Glucose-regulated protein 78 (GRP78) dissociates from protein kinase R-like endoplasmic reticulum kinase (PERK), ATF6, and IRE1 and ultimately initiates proapoptotic signaling pathways by activating CHOP. All three pathways of the UPR can induce CHOP activation. Phosphorylated eukaryotic initiation factor 2α (IF2α) can promote ATF4 expression and then activate the expression of the downstream protein CHOP and induce cell apoptosis. Furthermore, the translation of CHOP is regulated by ATF6. CHOP can increase the expression of Bim, death receptor 5 (DR5), Bax, and Bak and inhibit the expression of Bcl-2 to play a proapoptotic role. The IRE1 pathway and caspase-7 pathway can cause activation of caspase-12. Activated caspase-12 promotes the activation of caspase-3/9 and eventually leads to apoptosis. TRAF2 recruits and activates ASK1, which subsequently phosphorylates and activates JNK.