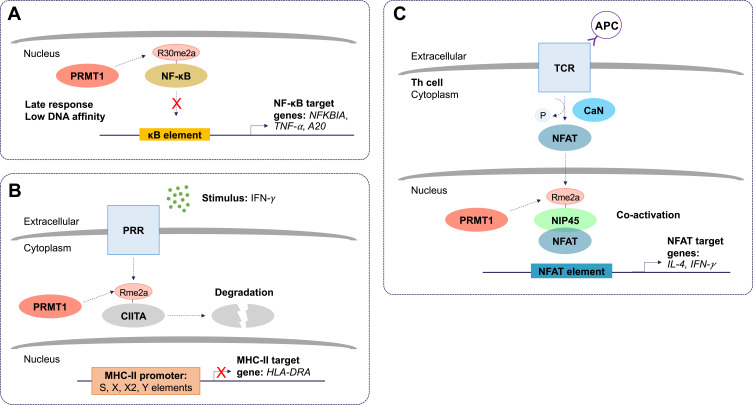
Figure 2.
The molecular and cellular function of PRMT1 during inflammation. (A) Protein arginine methyltransferase 1 (PRMT1) negatively regulates the nuclear factor kappa B (NF-κB) pathway. Asymmetric dimethylation (Rme2a) of the NF-κB subunit, RelA/p65, at R30, reduces its ability to bind to kappa B (κB) sites with the consensus sequence 5’-GGGRNYYYCC-3’, where R is an unspecified purine, Y is an unspecified pyrimidine, and N is any nucleotide. This prevents activation of promoters of NF-κB target genes. Asymmetric dimethylation of NF-κB is postulated to function as a late response in NF-κB activation. (B) PRMT1 suppresses class II trans-activator (CIITA)-mediated major histocompatibility complex II (MHC-II) transactivation. Pattern recognition receptor (PRR) stimulation by interferon-gamma (IFN-γ) results in asymmetric dimethylation of CIITA. This targets CIITA for degradation and prevents its translocation to the nucleus where it can stimulate the expression of MHC-II genes. (C) Asymmetric dimethylation of nuclear factor of activated T cells (NFAT)-interacting protein 45 kDa (NIP45) by PRMT1 positively regulates expression of NFAT target genes in T helper (Th) cells. T cell receptor (TCR) and antigen presenting cell (APC) ligation activates calcineurin (CaN). CaN dephosphorylates NFAT, allowing it to translocate to the nucleus. The interaction between NFAT and asymmetrically dimethylated NIP45 enhances the transcription of target genes.