Abstract
OBJECTIVE
To assess lifetime cardiovascular disease (CVD) risk by coronary artery calcium (CAC) score in individuals with diabetes from the Multi-Ethnic Study of Atherosclerosis (MESA) and compare risk with that in individuals without diabetes.
RESEARCH DESIGN AND METHODS
We developed a microsimulation model with well, diabetes, post-CVD, and death health states using multivariable time-dependent Cox regression with age as time scale. We initially used 10-year follow-up data of 6,769 MESA participants, including coronary heart disease (CHD) (n = 272), heart failure (n = 201), stroke (n = 186), and competing death (n = 619) and assessed predictive validity at 15 years. We externally validated the model in matched National Health and Nutrition Examination Survey (NHANES) participants. Subsequently, we predicted CVD risk until age 100 years by diabetes, 10-year pooled cohort equations risk, and CAC score category (0, 1–100, or 100+).
RESULTS
The model showed good calibration and discriminative performance at 15 years, with discrimination indices 0.71–0.78 across outcomes. In the NHANES cohort, predicted 15-year mortality risk corresponded well with Kaplan-Meier risk, especially for those with diabetes: 29.6% (95% CI 24.9–34.8) vs. 32.4% (95% CI 27.2–37.2), respectively. Diabetes increased lifetime CVD risk, similar to shifting one CAC category upward (from 0 to 1–100 or from 1–100 to 100+). Patients with diabetes and CAC score of 0 had a lifetime CVD risk that overlapped with that of individuals without diabetes who were at low 10-year pooled cohort equations risk (<7.5%).
CONCLUSIONS
Patients with diabetes carry a spectrum of CVD risk. CAC scoring may improve decisions for preventive interventions for patients with diabetes by better delineating lifetime CVD risk.
Introduction
The premise that coronary artery calcium (CAC) scoring by computed tomography (CT) enhances the effectiveness of periodic risk assessment for cardiovascular disease (CVD) is based predominantly on data from epidemiological studies and clinical trials. Epidemiological studies have shown CAC scores improve CVD risk prediction (1), and relevant clinical trials of pharmacological interventions have shown a clear association between predicted baseline risk and absolute risk reductions of CVD (2,3).
In individuals with diabetes, the utility of CVD risk assessment with CAC has been controversial and is not recommended by current American Heart Association (AHA)/American College of Cardiology (ACC) guidelines (4), because diabetes is considered a coronary heart disease (CHD) risk equivalent (i.e., a condition that puts individuals at high CVD risk independently of other risk factor levels). However, a low CAC score may indicate a much lower long-term CVD risk than a higher CAC score in individuals with diabetes. As such, CAC scoring could potentially also play an important role in decisions regarding preventive therapies for individuals with diabetes.
To extrapolate CVD risk based on longitudinal epidemiological data to the long term, individual-level state-transition or microsimulation models are useful. In addition, microsimulations of lifetime CVD outcomes can help determine the optimal strategy for CVD prevention, because preventive interventions are generally prescribed over an individual’s remaining lifetime. Predictions of lifetime CVD outcomes are, however, only useful for supporting clinical and public health decisions when the model that generates the predictions is credible.
The objectives of this article were to 1) develop and validate a microsimulation model using Multi-Ethnic Study of Atherosclerosis (MESA) data that include CAC scores in individuals with and without diabetes and 2) predict and describe lifetime cardiovascular risk by baseline CAC score as well as traditional CVD risk factor levels in MESA participants with and without diabetes.
Research Design and Methods
Study Overview
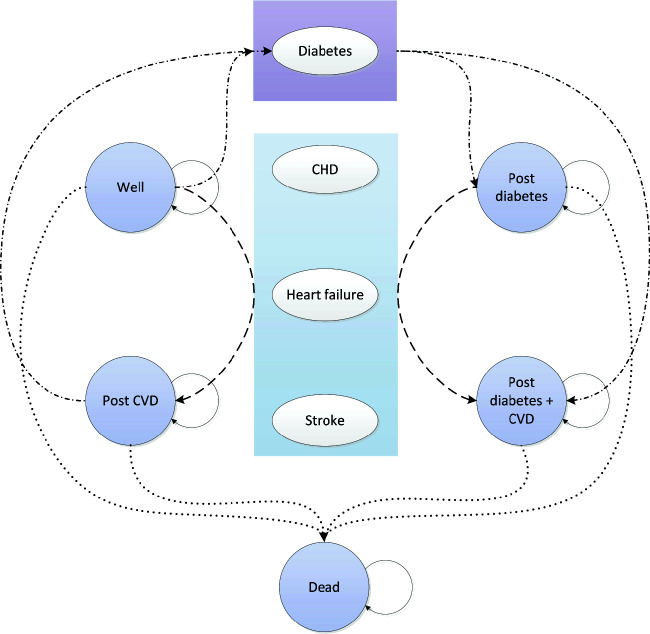
We developed a microsimulation state-transition model using R version 4.0.3 (R Foundation for Statistical Computing; https://www.r-project.org/) and TreeAge Pro 2021 software (TreeAge Software, Inc., Williamstown, MA). The model was structured to allow for modeling the following health states: well (defined as CVD and diabetes free), diabetes, post-CVD, post-CVD and diabetes, and death (Fig. 1). We initially derived probabilities for the state transitions using longitudinal MESA data with a 10-year follow-up duration. We subsequently tested model predictions for internal validity as well as predictive validity using extended 15-year follow-up data. After refitting the final model with the 15-year data, we determined external validity using the Public-Use Linked Mortality Files for the National Health and Nutrition Examination Survey (NHANES) 1999–2000 and 2001–2002 examination cycles.
Figure 1.
State-transition diagram.
Study Population for Model Development
Details on the study design of MESA have been published elsewhere (5). In brief, MESA was designed to evaluate risk factors of CVD in a multiethnic cohort of 6,814 U.S. individuals age 45–84 years without a history of clinical CVD at baseline. Participants had their baseline study visits between 2000 and 2002, during which CT CAC scanning was performed. Demographics, smoking status, medical history, and medication use were obtained by questionnaire. Physical examinations included measurement of height and weight and three measurements of resting blood pressure. Serum laboratory tests included lipids, fasting glucose, and creatinine. Diabetes was defined as fasting glucose ≥126 mg/dL, use of oral hypoglycemic medication and/or insulin, or self-reported physician diagnosis. CAC in Agatston units was measured twice for each participant either with electron-beam CT or multidetector row helical CT. CHD was defined as myocardial infarction or cardiac death. Stroke was defined by a documented focal neurological deficit lasting 24 h or until death or, if <24 h, a clinically relevant lesion on brain imaging. Heart failure required a combination of heart failure symptoms, physician diagnosis, and medical treatment.
After excluding participants with missing diabetes status (n = 24) and missing follow-up (n = 21), the final cohort for model development included 6,769 MESA participants (mean age 62 years; n = 853 with diabetes and n = 5,916 without diabetes at baseline) (Supplementary Table 1).
Estimation of Transition Probabilities and Parameter Uncertainty
To estimate transition probabilities over a long-term time horizon, we used time-dependent Cox regression of CHD (n = 272), heart failure (n = 201), stroke (n = 186), and competing death (n = 619), with chronological age as time scale. Hazard rates were then estimated by using the set of individuals who were at risk for the event of interest using attained age instead of their time on study, the conventional time scale. Use of chronological age as time scale is attractive, because hazard rates and cumulative incidences can then be estimated across the entire range of failure ages, spanning from 47.1 to 97.4 years in the 6,769 MESA participants. In addition, it may avoid misspecification of the baseline age effect when time on study is used, which is especially relevant when hazard rates of events of interest heavily depend on age (6). We assumed hazard ratios (HRs) of CAC score, diabetes, and other baseline predictors were constant across an individual’s age, and no birth cohort effects were present.
Event rates that defined transition probabilities were individualized using baseline predictor variables (sex, ethnicity, smoking, blood pressure, antihypertensive medication use, lipids, statin use, BMI, diabetes, and CAC score) and were further modified with incident diabetes (n = 769) and cardiovascular events as time-dependent variables. Calculation of individualized event rates involved multiplying baseline hazard rates by each individual’s linear predictor defined by applicable HRs of selected predictor variables and predictor values. Predictors were selected from full models including all candidate predictors (7,8) in a backward stepwise manner using the Akaike information criterion for selection. We explored nonlinear associations of BMI by including quadratic terms and interactions of systolic blood pressure by treatment with antihypertensive medication and baseline diabetes with sex. Nonlinear and interaction terms were selected based on a likelihood ratio test with P < 0.01 as criterion.
Baseline survival and cumulative hazard functions were estimated from age 45 until 100 years (Supplementary Fig. 1) and were smoothed and extrapolated beyond the maximum age at which an event occurred by restricted cubic spline functions, which predicted log cumulative hazard rates by log age. We implemented a fixed 1-year cycle length in the state-transition model. Because occurrence of only one event was possible within each cycle, we accounted for competing risks by assuming constant 1-year hazard rates within each model cycle. For case fatality of CHD, stroke, and heart failure, we fitted logistic regression models with 30-day mortality as outcome and age as predictor (Supplementary Table 2).
To account for parameter uncertainty, Cox and logistic regression models and cumulative hazard functions were estimated in 1,000-bootstrap data sets. Single imputation within each bootstrap was performed to handle missing predictors (9). For the imputations, we used a multivariable flexible additive model using predictive mean matching and included status variables and the Nelbbson-Aalen estimator of the cumulative hazards for CVD events and competing death. Microsimulations were based on the 1,000-bootstrap parameter sets, and 95% uncertainty intervals (95% UIs) of model outcomes were calculated using the percentile method.
Model Validation
To increase credibility of microsimulations, we applied three types of model validation. First, we assessed the ability of the model to reproduce outcomes as observed in the MESA data by comparing model predictions with observed 10-year cumulative incidences and estimations of discriminative performance using the Harrell concordance statistic (C-statistic). To calculate C-statistics, predictions were acquired by simulation of MESA participants and estimation of 10-year risk for each simulated individual using 200 random walks.
Second, to obtain confidence about the ability to extrapolate outcomes beyond the follow-up period of the underlying study data, we assessed predictive validity. In this validation step, we compared 15-year model predictions with cumulative incidences obtained from data on the 6,769 MESA participants with extended 15-year follow-up. Again, we evaluated the C-statistics for discriminative ability of model predictions. After assessment of predictive validity, a final microsimulation model was refit on the 15-year follow-up data. At the end of this research, new MESA data became available, allowing for follow-up of events through an 18-year period. However, given the increasing uncertainty of estimates beyond 15-year follow-up, we decided to include analyses of predictive validity through 18-year follow-up as supplemental analyses.
Lastly, to evaluate the extent to which model predictions are reproducible in independent populations, we compared predictions by the final model with observations from another data source (external validity). We selected 4,422 NHANES participants from examination cycles 1999–2000 and 2001–2002 who met MESA inclusion criteria. Because NHANES does not include CT CAC measurements, we used a matching procedure to reproduce likely predictions of outcomes obtained from similar MESA participants, assuming similar CAC scores conditional on risk factors available in both MESA and NHANES. To improve comparability in the case mix, for an assessment of reproducibility of model predictions, we matched one to one for age, sex, race/ethnicity, education (less than high school, high school, some college, or college or higher), BMI, and 10-year atherosclerotic CVD risk as estimated by pooled cohort equations (10) after stratifying for diabetes status. We then simulated 15-year mortality using the matched MESA participants and compared with observed Kaplan-Meier estimates in matched NHANES participants.
Lifetime Predictions of Cardiovascular Outcomes
We subsequently ran the final model until age 100 years for the 6,769 MESA participants and stratified predicted risks of CVD by baseline age, sex, diabetes, 10-year pooled cohort equations risk, and CAC score category: 0, 1–100, and 100+, as recommended in the recent AHA/ACC guidelines (4). We also plotted distributions of predicted cumulative incidence of CVD (i.e., CHD, heart failure, or stroke).
Institutional Review Board Approval
All MESA participants gave informed consent, and the study protocol was approved by the institutional review board (IRB) at each site. The Mount Sinai Hospital IRB deemed the study protocol for our modeling study as exempt from IRB review.
Results
Model Development
MESA participants with diabetes at baseline (n = 853) were older (median age 66 vs. 62 years), more likely to be of African American or Hispanic ethnicity (70 vs 47%), and more often had high BMI (median BMI 29.8 vs. 27.3 kg/m2), high blood pressure (median systolic blood pressure 131 vs. 123 mmHg), and a non-zero CAC score (63 vs. 48%) than participants without diabetes (n = 5,916) (Supplementary Table 1). During 10-year follow-up, 272 CHD events, 201 heart failures, 186 strokes, and 619 competing deaths occurred. At 15 years, these increased to 419, 275, 288, and 1,073, respectively.
Female sex, Asian ethnicity, higher HDL cholesterol, and statin use were generally associated with lower cardiovascular and mortality rates. Current smoking, higher BMI, hypertension, higher total cholesterol, diabetes, and higher CAC score at baseline were associated with higher adverse event rates. In addition to baseline diabetes (HR 1.58; 95% UI 1.27–1.97), incident diabetes (HR 2.03; 95% UI 1.34–3.10) was also independently associated with competing mortality, but both HRs were lower than that for incident CVD (HR 3.11; 95% UI 2.47–3.92) (Supplementary Table 3). None of the evaluated interaction terms were retained in any of the models.
Model Validation
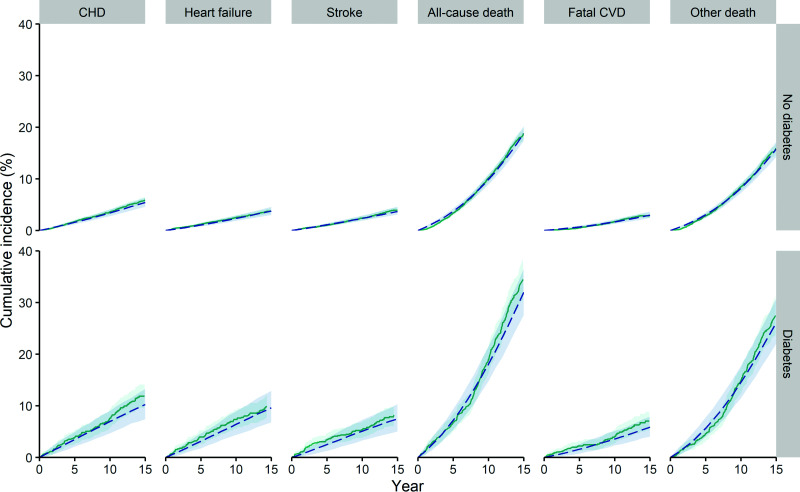
The model showed good internal and predictive validity of cumulative incidences across the outcomes in the MESA cohort through 15-year follow-up (Fig. 2), as well as through 18-year follow-up (Supplementary Fig. 2). Also, discriminative ability at 10, 15, and 18 years was good for both nonfatal and fatal outcomes (Supplementary Table 4). Odds ratios and HRs from the final microsimulation model fitted using 15-year follow-up data changed very little from those obtained using 10-year follow-up data (Supplementary Tables 5 and 6).
Figure 2.
Cumulative incidence curves of CHD, stroke, heart failure, all-cause mortality, fatal CVD, and other mortality for internal and predictive validity. Model predictions (dashed blue line) and MESA observations (solid green line), with 95% UIs indicated by gray shaded areas.
In the matched NHANES cohort (n = 353 with diabetes and n = 3610 without diabetes) (Supplementary Table 7), 690 deaths occurred during 15 years of follow-up. Supplementary Figure 3 shows that model predictions of mortality risk corresponded well with observed Kaplan-Meier estimates, especially for those with diabetes (29.6%; 95% CI 24.9–34.8 vs. 32.4%; 95% CI 27.2–37.2, respectively).
Lifetime CVD Risk Predictions
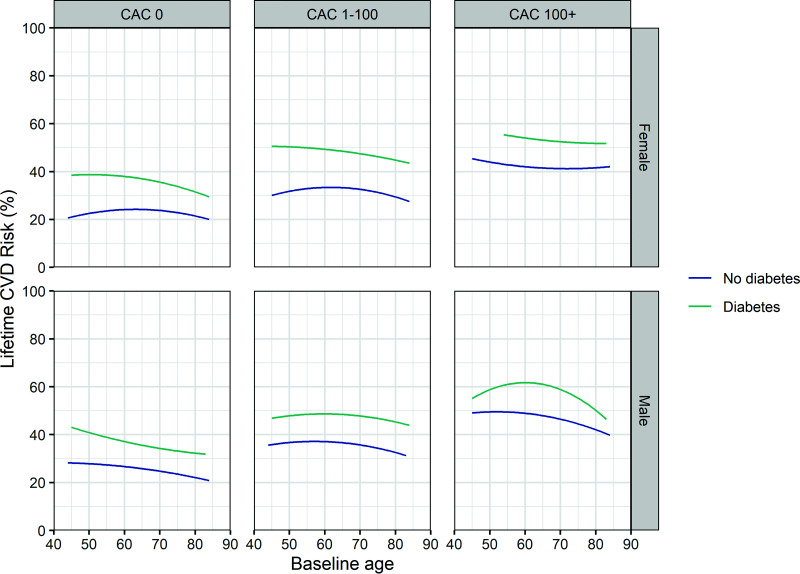
The distribution of microsimulations of cumulative cardiovascular risk until age 100 years among 6,769 MESA participants demonstrated that those with diabetes overall had a higher lifetime CVD risk (mean 46.5%; median 45.5%; interquartile range [IQR] 36.5–55.5) than those without diabetes (mean 31.3%; median 29.5%; IQR 22.0–39.0), with most participants with diabetes having a lifetime CVD risk >20% (Supplementary Fig. 4). Microsimulations also revealed that male MESA participants generally had a higher lifetime CVD risk than female participants (37.1%; median 35.5%; IQR 27.0–46.0 vs. 29.8%; median 28.0%; IQR 20.5–37.0) (Fig. 3).
Figure 3.
Microsimulations of the lifetime cumulative incidence of CVD by baseline age, sex, diabetes, and CAC score. CVD is defined as a composite end point of CHD, heart failure, and stroke. Curves depict mean lifetime CVD risk predictions by baseline age among 6,769 MESA participants stratified by baseline diabetes, sex, and CAC score category.
Furthermore, the model predicted higher lifetime CVD risk for those with a baseline CAC score >0. A one-category shift to a more severe level of CAC (e.g., from 0 to 1–100 or from 1–100 to 100+) appeared to increase lifetime CVD risk similarly to the increase associated with presence of baseline diabetes (Fig. 3). For example, lifetime CVD risk in individuals with diabetes and a 0 CAC score at baseline was similar to the risk in those without diabetes and a CAC score 1–100 (36.8%; median 36.8%; IQR 30.5–43.5 vs. 34.2%; median 33.5%; IQR 27.5–40.5). Of note, female participants generally had an elevated baseline CAC score (1–100 or 100+) at an older age than men (67.7 vs. 65.4 years).
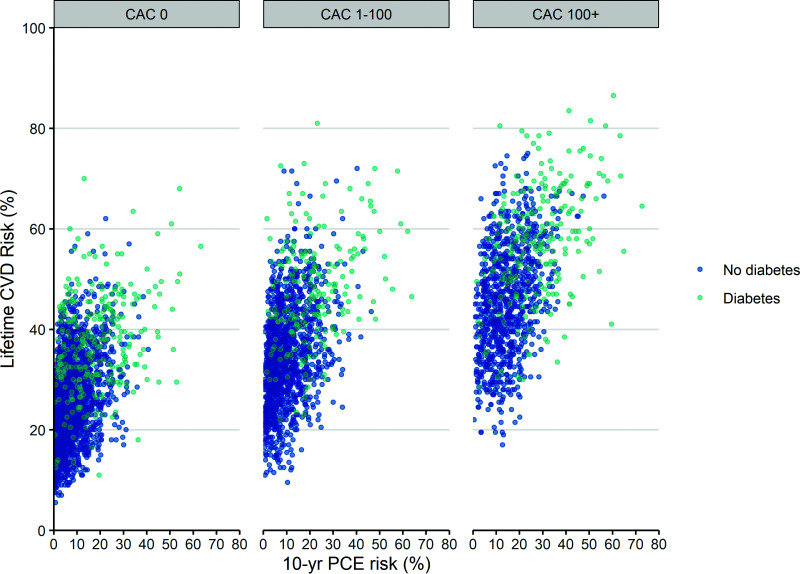
Lifetime CVD risk of patients with diabetes, 10-year pooled cohort equations risk <7.5%, and CAC score of 0 overlapped with those of individuals with a similar profile, but without diabetes (Fig. 4). For example, ∼16% of such low-risk patients with diabetes had a lifetime CVD risk ≤75th percentile lifetime CVD risk of low-risk individuals without diabetes.
Figure 4.
Microsimulations of the lifetime cumulative incidence of CVD by baseline diabetes, 10-year CVD risk, and CAC score. CVD is defined as a composite end point of CHD, heart failure, and stroke. Lifetime CVD risk predictions by the model are depicted for 6,769 MESA participants stratified by 10-year CVD disease risk based on pooled cohort equations (PCE), diabetes, and CAC score category.
Conclusions
In this study, we developed and validated a microsimulation model using MESA data and subsequently assessed the distribution of lifetime CVD risk for 6,769 MESA participants according to their baseline diabetes and CAC score status. Our microsimulations revealed several important findings. First, our results reinforce the impact of diabetes and CAC on the lifetime risk of CVD. Individuals with diabetes and/or non-zero CAC score generally carry a much higher lifetime CVD risk than individuals without, even though both risk factors were also associated with higher competing risk of dying as a result of causes other than CVD. Second, a one-category increase in CAC score (from 0 to 1–100 or from 1–100 to 100+) was associated with an increase in lifetime CVD risk similar to that associated with the presence of diabetes. Lastly, a nonnegligible proportion of patients with diabetes, a low 10-year pooled cohort equations risk, and 0 CAC score had lifetime CVD risk similar to low-risk individuals without diabetes.
CAC scoring has generally not been considered relevant to preventive treatment decisions in individuals with diabetes, in part because diabetes itself carries increased cardiovascular risk, in some analyses equivalent to the risk of subsequent events following CHD (CHD risk equivalent). Per current AHA/ACC guidelines, routinely assessing 10-year cardiovascular risk with pooled cohort equations and CAC scoring for initiation of statins in patients with diabetes is therefore not recommended (4). However, more recent studies have challenged this concept of diabetes as a CHD risk equivalent. For example, a study conducted within the Kaiser patient population showed that diabetes conveys an excess risk lower than expected. In this population, CHD was associated with an HR of 2.8 (95% CI 2.7–2.85) for subsequent CHD events versus an HR of 1.7 (95% CI 1.66–1.74) for diabetes alone (11). As such, it is very likely that individuals with diabetes carry a spectrum of CVD risk and that not all individuals with diabetes are at high absolute CVD risk. Indeed, our microsimulations demonstrate that lifetime CVD risk varies substantially in individuals with diabetes and that baseline CAC score is an important predictor of the variation.
Various previously published studies have forecasted long-term cardiovascular outcomes including CHD, stroke, and heart failure based on population-based cohort data (12–19). Predictors considered in these prognostic studies generally include risk factors that are routinely available, but none of the studies offer long-term cumulative incidence estimates accounting for CAC score. However, CAC scores are particularly valuable for estimation of long-term CVD risk, because they incorporate the impact of cumulative exposure to cardiovascular risk factors. Moreover, CAC scores also play a role in predicting non-CVD outcomes, including cancer, chronic kidney disease, chronic obstructive pulmonary disease, hip fracture, and dementia (20,21), with associated competing risks of death that may become important when estimating the cumulative incidence of CVD events over a longer time horizon.
Our findings of men having an elevated CAC score at a younger age than women as well as higher lifetime CVD risk may indicate that the impact of CAC on CVD incidence outweighs its impact on competing death risk and the longer life expectancy of women. In several previous analyses of lifetime CVD risk estimates, risk in men was found to be higher, even though end point definitions for CVD varied. For example, predictions based on the QRISK cohort including patients from QResearch general practices in England (18) demonstrated men had a higher CVD lifetime risk than women. Also, in a pooled analysis of data from five National Heart, Lung, and Blood Institute–funded community-based cohorts, lifetime CVD risk was estimated to be slightly higher for men (60.3 vs. 55.6% at age 45 years) (14,15). However, in an analysis of lifetime CVD risk using data from the population-based Rotterdam study (22), estimated lifetime CVD risks of men and women were quite similar (at age 55 years, 67.1% for men and 66.4% for women). The Rotterdam study population is known to be healthier (23), which may have resulted in less prominent absolute CVD risk differences. The CVD end point used by Leening et al. (22) included surgical or percutaneous coronary revascularization procedures, transient ischemic attack, and carotid revascularization procedures. Women can have similar or even higher rates of angina and transient ischemic attacks compared with men, especially in low-risk populations (24,25). Of note, none of the aforementioned analyses considered heart failure as outcome for estimating lifetime cardiovascular risk, although prior analyses have shown similar risk by sex (13).
Strengths and Limitations
In comparison with the previously published studies, our study adds new information resulting from the incorporation of CAC scores and use of more recent population-based cohort data. Second, the validity of our microsimulations was tested against extended follow-up data as well as independent external data, increasing the plausibility of our findings. Despite these strengths, some limitations need to be considered.
First, we extrapolated predictions to a lifetime horizon, whereas the final model was developed using data with a maximum 15-year follow-up duration. Developing predictions on longer follow-up data (e.g., 30 years) would allow for a more comprehensive evaluation of long-term risk. However, this approach may be vulnerable to chronological changes in CVD event rates and associated risk factors, which are less likely to affect predictive validity if more recent and thus shorter follow-up data are used. Because MESA is ongoing and longer follow-up is being obtained, we will be able to test validity of our extrapolations further.
Second, NHANES does not contain information on nonfatal CVD outcomes, and therefore, validity of these outcomes could not be evaluated externally. An independent external validation of our predictions of CVD rates would need to be conducted in other population-based cohort studies, but this was beyond the scope of the current study. Also, the fact that our model proved to be valid for predicting mortality in individuals who matched eligible NHANES participants does not automatically imply that it will be valid in other populations as well. Because NHANES data sets do not contain data on CT CAC scoring, matching was required to allow a fair comparison of predicted outcomes with those observed. We intend to validate the model with other data as well when available.
Third, predictions of event rates could be further individualized for patients with diabetes by including parameters of disease severity, such as glycated hemoglobin (HbA1c) and duration of diabetes. For example, Malik et al. (26) showed that CVD event rates may be higher in those with diabetes for ≥10 years when CAC score is ≥400. However, HbA1c was not assessed at baseline in MESA, and duration of diabetes was not available for the entire group of patients with diabetes. In addition, the latter variable may be misclassified as a result of recall problems.
Finally, CAC categories were defined as 0, 1–100, and 100+ based on ACC/AHA guideline recommendations (4). As an alternative to categorization by absolute CAC score, CAC score classification by age, sex, and ethnicity percentiles is endorsed. However, CVD risk prediction based on categorization by absolute score has been demonstrated to perform better (27) and is more widely used, rendering our findings to be more generalizable.
Implications for Clinical Practice and Further Research
Our microsimulations reaffirm recent insights that diabetes cannot ubiquitously be considered to indicate a CVD risk equal to that of having a history of CHD. Prior analyses of MESA data have already shown that incidence rates of CHD and atherosclerotic CVD are very low in those with diabetes when CAC score is 0 (<10 per 1,000 person-years) (26,28). Our study provides further insights by showing how lifetime CVD risk in patients with diabetes depends on baseline CAC score, in addition to the individual’s age and conventional cardiovascular risk factors. Health benefits of preventive interventions can be extrapolated from these long-term risk predictions and expressed in terms of absolute risk reduction (number needed to prevent) over a preset time horizon or as gain in disease-free life expectancy. Such estimates may be used as communication tools for shared decision making, as proposed by others (17).
The impact of incorporating CAC scoring into preventive treatment decision making may be predominantly defined by the likelihood of finding a 0 CAC score in those who also have favorable levels of other cardiovascular risk factors. In some patients with diabetes and 0 CAC score, aggressive preventive interventions could potentially be safely deferred or omitted, because absolute treatment benefit will likely be small, such as that also expected in individuals without diabetes, low 10-year pooled cohort equations risk, and 0 CAC score (Fig. 4). Other motivations to use CAC scoring in patients with diabetes would be to guide decisions about intensifying pharmacological treatment to increase cardiovascular benefits and discontinuing treatment following adverse effects (29). Furthermore, a meta-analysis of six prospective cohort studies suggests that detection of non-zero CAC scores may serve as a motivational tool for patients to improve lipid-lowering medication adherence, exercise, and dietary habits (30).
However, the expected change in remaining lifetime CVD rates may not be the sole driver for treatment decisions at the individual level (31). From the individual’s perspective, for example, risk and treatment preferences, as well as costs of CT when not (fully) reimbursed, may also play important roles. For decision making from a broader population perspective, however, the costs to the health care sector and society should be weighed against the expected health benefits. Valuation of health following interventions and CVD events may then also need to be placed in the context of societal preferences. Whether periodic CVD risk assessment including CAC scoring is valuable for patients with diabetes should therefore be addressed by rigorous decision analytical and cost-effectiveness studies, as undertaken previously for individuals without diabetes (1).
In conclusion, patients with diabetes carry a spectrum of CVD risk. CAC scoring may improve decisions for preventive interventions for patients with diabetes by better delineating lifetime CVD risk.
Article Information
Acknowledgments. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. This article was prepared using MESA research materials obtained from the National Heart, Lung, and Blood Institute and has been reviewed by MESA for scientific content and consistency of data interpretation with previous MESA publications. A full list of participating MESA investigators and institutions can be found at https://www.mesa-nhlbi.org.
Funding. MESA was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute, National Institutes of Health, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences. B.S.F., M.G.M.H., U.M., W.M., and K.E.F. were supported by American Diabetes Association grant 1-18-ICTS-041.
The American Diabetes Association had no role in the design or conduct of the study, collection, management, analysis, or interpretation of the data, preparation, review, or approval of the manuscript, or the decision to submit the manuscript for publication.
Duality of Interest. M.G.M.H. receives royalties from Cambridge University Press for a textbook on medical decision making, reimbursement of expenses from the European Society of Radiology (ESR) for work on ESR guidelines for imaging referrals, reimbursement of expenses from the European Institute for Biomedical Imaging Research for membership on the scientific advisory board, and additional research funding from the Netherlands Organization for Health Research and Development, the German Innovation Fund, a Netherlands Educational Grant (Studie Voorschot Middelen), and the Gordon and Betty Moore Foundation. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. B.S.F. and M.G.M.H. performed statistical analysis. K.E.F. obtained funding. All authors contributed to study conception and design, acquisition, analysis, or interpretation of data, critical revision of the manuscript for important intellectual content, and provision of administrative, technical, or material support. B.S.F. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the American Diabetes Association’s 79th Scientific Sessions, 7–11 June 2019, and the Society of Medical Decision Making’s 42nd Annual North American Meeting, 6–27 October 2020.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.19082711.
References
- 1. Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol 2018;72:434–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016;388:2532–2561 [DOI] [PubMed] [Google Scholar]
- 3. Blood Pressure Lowering Treatment Trialists’ Collaboration . Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet 2014;384:591–598 [DOI] [PubMed] [Google Scholar]
- 4. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;140:e596–e646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–881 [DOI] [PubMed] [Google Scholar]
- 6. Thiébaut AC, Bénichou J. Choice of time-scale in Cox’s model analysis of epidemiologic cohort data: a simulation study. Stat Med 2004;23:3803–3820 [DOI] [PubMed] [Google Scholar]
- 7. Kronmal RA, McClelland RL, Detrano R, et al. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2007;115:2722–2730 [DOI] [PubMed] [Google Scholar]
- 8. Lehmann N, Erbel R, Mahabadi AA, et al.; Heinz Nixdorf Recall Study Investigators . Value of progression of coronary artery calcification for risk prediction of coronary and cardiovascular events: result of the HNR study (Heinz Nixdorf Recall). Circulation 2018;137:665–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brand J, van Buuren S, le Cessie S, van den Hout W. Combining multiple imputation and bootstrap in the analysis of cost-effectiveness trial data. Stat Med 2019;38:210–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al.; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(Suppl. 2):S49–S73 [DOI] [PubMed] [Google Scholar]
- 11. Rana JS, Liu JY, Moffet HH, Jaffe M, Karter AJ. Diabetes and prior coronary heart disease are not necessarily risk equivalent for future coronary heart disease events. J Gen Intern Med 2016;31:387–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lloyd-Jones DM, Leip EP, Larson MG, et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation 2006;113:791–798 [DOI] [PubMed] [Google Scholar]
- 13. Huffman MD, Berry JD, Ning H, et al. Lifetime risk for heart failure among white and black Americans: cardiovascular lifetime risk pooling project. J Am Coll Cardiol 2013;61:1510–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wilkins JT, Ning H, Berry J, Zhao L, Dyer AR, Lloyd-Jones DM. Lifetime risk and years lived free of total cardiovascular disease. JAMA 2012;308:1795–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Berry JD, Dyer A, Cai X, et al. Lifetime risks of cardiovascular disease. N Engl J Med 2012;366:321–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Berkelmans GFN, Gudbjörnsdottir S, Visseren FLJ, et al. Prediction of individual life-years gained without cardiovascular events from lipid, blood pressure, glucose, and aspirin treatment based on data of more than 500 000 patients with type 2 diabetes mellitus. Eur Heart J 2019;40:2899–2906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jaspers NEM, Blaha MJ, Matsushita K, et al. Prediction of individualized lifetime benefit from cholesterol lowering, blood pressure lowering, antithrombotic therapy, and smoking cessation in apparently healthy people. Eur Heart J 2020;41:1190–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hippisley-Cox J, Coupland C, Robson J, Brindle P. Derivation, validation, and evaluation of a new QRISK model to estimate lifetime risk of cardiovascular disease: cohort study using QResearch database. BMJ 2010;341:c6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pencina MJ, D’Agostino RB Sr, Larson MG, Massaro JM, Vasan RS. Predicting the 30-year risk of cardiovascular disease: the Framingham Heart Study. Circulation 2009;119:3078–3084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Handy CE, Desai CS, Dardari ZA, et al. The association of coronary artery calcium with noncardiovascular disease: the Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc Imaging 2016;9:568–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fujiyoshi A, Jacobs DR Jr, Fitzpatrick AL, et al. Coronary artery calcium and risk of dementia in MESA (Multi-Ethnic Study of Atherosclerosis). Circ Cardiovasc Imaging 2017;10:e005349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leening MJ, Ferket BS, Steyerberg EW, et al. Sex differences in lifetime risk and first manifestation of cardiovascular disease: prospective population based cohort study. BMJ 2014;349:g5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leening MJ, Heeringa J, Deckers JW, et al. Healthy volunteer effect and cardiovascular risk. Epidemiology 2014;25:470–471 [DOI] [PubMed] [Google Scholar]
- 24. Virani SS, Alonso A, Aparicio HJ, et al.; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation 2021;143:e254–e743 [DOI] [PubMed] [Google Scholar]
- 25. Vyas MV, Silver FL, Austin PC, et al. Stroke incidence by sex across the lifespan. Stroke 2021;52:447–451 [DOI] [PubMed] [Google Scholar]
- 26. Malik S, Zhao Y, Budoff M, et al. Coronary artery calcium score for long-term risk classification in individuals with type 2 diabetes and metabolic syndrome from the Multi-Ethnic Study of Atherosclerosis. JAMA Cardiol 2017;2:1332–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Budoff MJ, Nasir K, McClelland RL, et al. Coronary calcium predicts events better with absolute calcium scores than age-sex-race/ethnicity percentiles: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2009;53:345–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Malik S, Budoff MJ, Katz R, et al. Impact of subclinical atherosclerosis on cardiovascular disease events in individuals with metabolic syndrome and diabetes: the Multi-Ethnic Study of Atherosclerosis. Diabetes Care 2011;34:2285–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nasir K, Cainzos-Achirica M. Role of coronary artery calcium score in the primary prevention of cardiovascular disease. BMJ 2021;373:n776. [DOI] [PubMed] [Google Scholar]
- 30. Gupta A, Lau E, Varshney R, et al. The identification of calcified coronary plaque is associated with initiation and continuation of pharmacological and lifestyle preventive therapies: a systematic review and meta-analysis. JACC Cardiovasc Imaging 2017;10:833–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hussain A, Ballantyne CM, Nambi V. Zero coronary artery calcium score: desirable, but enough? Circulation 2020;142:917–919 [DOI] [PubMed] [Google Scholar]