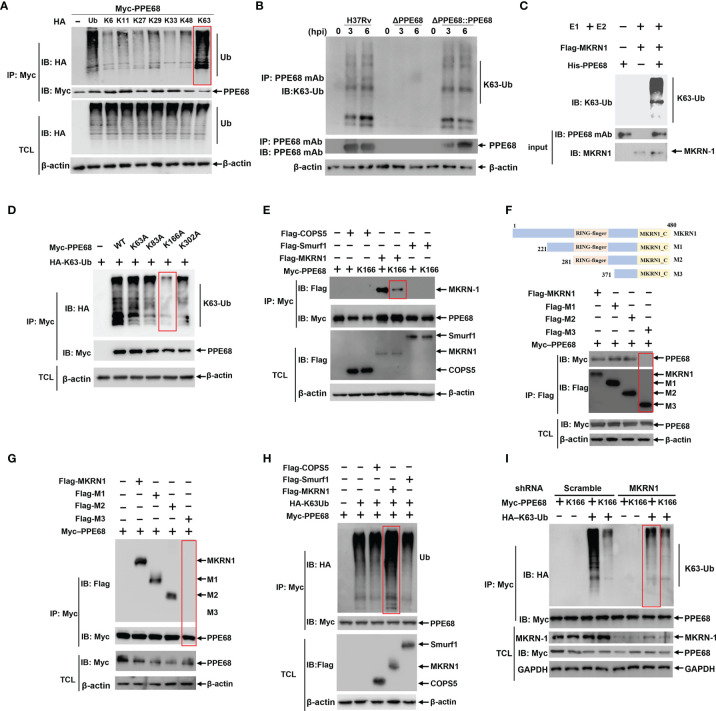
Figure 2.
MKRN1 interacts with PPE68 and promotes the attachment of K63-linked ubiquitin chains to K166 of PPE68. (A, D-I) HEK293T cells were transfected with the indicated plasmids for 24 h, and then cell lysates were analyzed by immune-precipitation (IP) and Western blot with anti-HA, anti-Myc and anti-Flag antibodies. PPE68 ubiquitination status (A). PPE68 critical ubiquitination site at K166 (D). MKRN1 interacts with PPE68, but not PPE68 K166 mutant (E). MKRN1 critical binding domain for PPE68 (F, G). MKRN1 overexpression promotes ubiquitination for PPE68 (H). PPE68 could not be ubiquitinated after silencing MKRN1 (I). (B) PPE68 protein in H37Rv and RvΔPPE68::PPE68 could be ubiquitinated. BMDMs were infected with H37Rv, RvΔPPE68 or RvΔPPE68::PPE68 (MOI=10) for the indicated time course, and then cell lysates were analyzed by IP with an anti-PPE68 mAb and Western blot with an anti-PPE68 mAb and anti-K63 Ub antibodies. (C) Western blot analysis of PPE68 ubiquitination in vitro. The purified His-PPE68 and Flag-MKRN1 proteins, and the ubiquitin, E1 and E2 ligases were mixed for ubiquitination assay. The mixtures were incubated at 37°C for 90 min and analyzed via Western blot with an anti-K63 Ub antibody. TCL, total cell lysates.