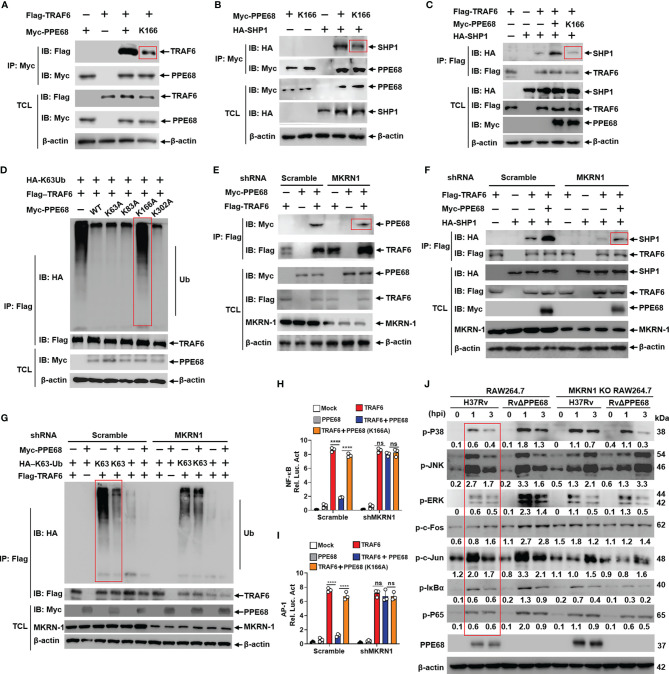
Figure 4.
PPE68 K166 ubiquitination interacts with TRAF6 and SHP1, and then inhibits TRAF6-NF-κB/AP-1 signaling. (A-G) HEK293T cells were transfected with the indicated plasmids for 24 h, and then cell lysates were analyzed by IP and Western blot with anti-HA, anti-Myc and anti-Flag antibodies. PPE68, but not PPE68 K166 mutant, could interact with TRAF6 (A) and SHP1 (B). PPE68, but not PPE68 K166 mutant, overexpression could promote the interaction between TRAF6 and SHP1 (C), and TRAF6 ubiquitination (D). PPE68 interaction with TRAF6 was reduced after MKRN1 silencing (E). After MKRN1 silencing, PPE68 overexpression could not promote the interaction between TRAF6 and SHP1 (F), and could not inhibit TRAF6 K63 ubiquitination, either (G). (H, I) After MKRN1 silencing, PPE68, but not PPE68 K166 mutant, could not inhibit TRAF6-driven NF-κB (H) and AP-1 (I) activation. Dual luciferase reporter assay of the effects of PPE68 or PPE68(K166A) and TRAF6 on the NF-κB (H) or AP-1 (I) activation in HEK293T cells co-transfected with shMKRN1 and Scramble for 24 (h) (J) H37Rv PPE68 inhibits NF-κB/MAPK signaling molecules phosphorylation dependent on MKRN1. Western blot analysis of phosphorylated P38, JNK, ERK, c-Fos, c-Jun, P65, IκBα and P65 and total β-actin (loading control throughout) in WT and MKRN1 KO RAW264.7 cells infected with H37Rv or RvΔPPE68 for the indicated time. Densitometry quantification as shown in panel (J) was analyzed based on β-actin by ImageJ. TCL, total cell lysates. The data are expressed as the mean ± SD of three independent replicates for (H, I) Two tailed unpaired t test was used to calculate statistical significance. p > 0.05, not significant (ns); ****p < 0.0001.