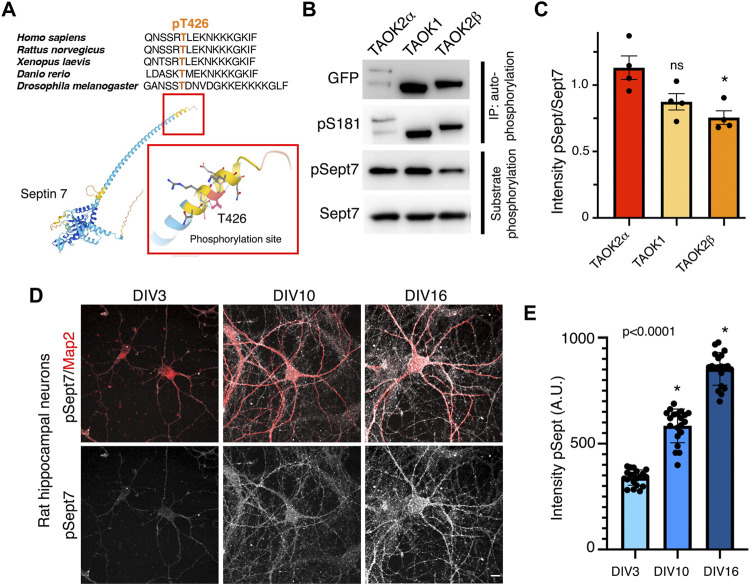
FIGURE 1.
TAO kinases phosphorylate the C-terminal tail of Sept7 during neuronal development. (A) Top: multiple sequence alignment of the C-terminal tail of Sept7 demonstrating its evolutionarily conserved sequence including the phosphorylation site. The phosphorylated residue T426 is shown in orange. Bottom: AlphaFold2.0 predicted protein structure of Sept7. Magnified inset depicts the T426 phosphorylation site (red) in the C terminal tail of Sept7. (B) Western blot shows kinase activity of sfGFP-TAOK2α, sfGFP-TAOK1, or sfGFP-TAOK2β expressed in HEK293T cells, immunoprecipitated with GFP antibody, and incubated with purified GST-Sept7 C-terminal tail (321–438 amino acids) for an in vitro kinase reaction. Substrate phosphorylation was determined by probing for phosphorylated Sept7 using the pT426 antibody. Total Sept7 was detected by antibody against GST tag. TAO kinase autophosphorylation was determined by probing for phosphorylated S181 residue. (C) Quantification of protein band intensity of Western blot in (B). Bar graph displays the ratio of phosphorylated Sept7 band intensity to GST band intensity for each TAO kinase construct in which no significant difference was observed between TAOK2α and TAOK1 kinases. Error bars represent standard error of mean from n = 4 experiments. (D) Rat hippocampal neurons at DIV3, 10, and 16 were fixed and immunostained for endogenous phosphorylated Sept7 T426 and dendritic protein Map2. Scale bar represents 10 μm. (E) Mean fluorescence intensity of phosphorylated Sept7 T426 at distinct stages of neuronal development DIV3, 10, and 16 are plotted. Error bars represent standard error of mean for n = 20 neurons from three different experiments. Dunnett’s multiple comparison shows significant changes in pSept7 levels between each stage.