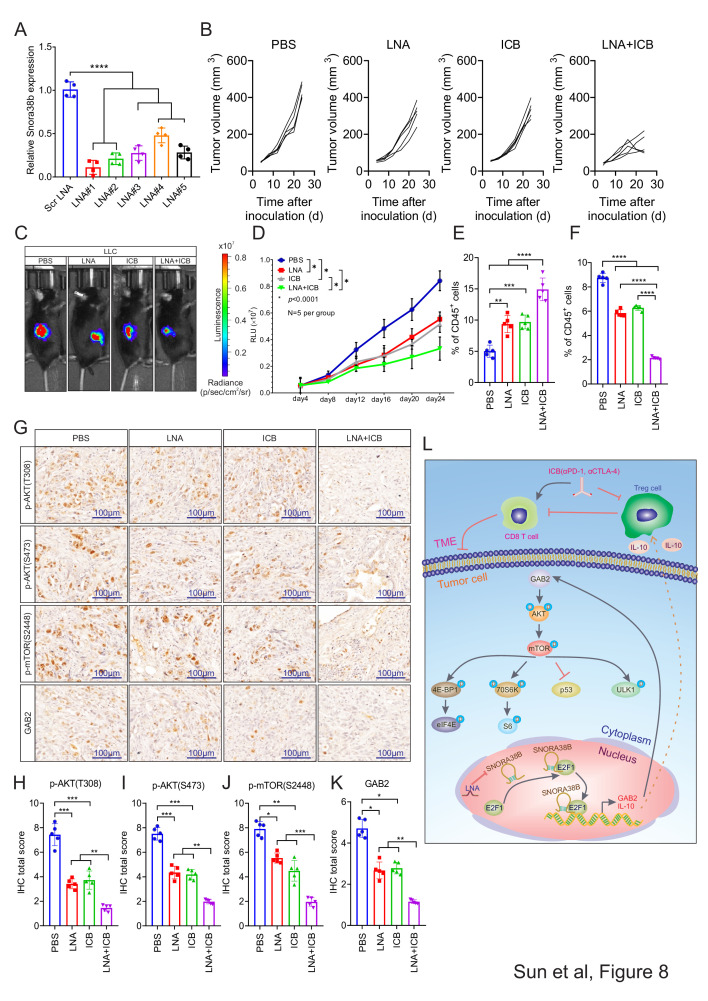
Figure 8.
SNORA38B LNAs sensitized NSCLC to ICBs treatment. (A) Transfection efficiency of LNAs in LLC cells. (B) Tumor volume examined in LLC derived tumors in tumor burden C57BL/6J mice according to formula 0.5×L×W2. n=5 per group. (C–D) Representative images and quantification of BLI in the luciferase reporter gene labeled LLC tumor regions in C57BL/6J mice treated with PBS, LNA, ICB or LNA+ICB. Signals were recorded every 4 days before reaching the observation endpoint. n=5 per group. (E–F) Flow cytometry detection of CD3+CD8+ T cells, CD4+FOXP3+ Tregs and their percentage of CD45+ cells isolated from LLC cells injected C57BL/6J mice treated with PBS, LNA, ICB or LNA+ICB. (G) IHC staining of AKT/mTOR/GAB2 signing pathway with the isolated burden tumors from C57BL/6J mice treated with PBS, LNA, ICB or LNA+ICB. Bar=100 µm. (H–K) Quantification of IHC staining of AKT/mTOR/GAB2 signing pathway. (L) Basic summary of the carcinogenic mechanism of SNORA38B in NSCLC. SNORA38B directly bound to the transcription factor E2F1, then accelerated the transcription of downstream GAB2 gene, activated AKT/mTOR pathway and its downstream effectors to perform cancer-promoting effects. Besides, SNORA38B recruited Tregs to mediate immunosuppression by promoting the secretion of IL-10. Knockdown of SNORA38B using LNAs could sensitize NSCLC to ICB treatment. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. Assays were conducted in triple. Means±SD was shown. Statistical analysis was subjected to one-way analysis of variance. Comparison between-group using LSD method. BLI, bioluminescence imaging; CTLA-4, cytotoxic T-lymphocytes-associated protein 4; E2F, E2F transcription factor; GAB2, GRB2-associated-binding protein 2; ICB, immune checkpoint blockade; IHC, immunohistochemistry; IL, interleukin; LLC, murine Lewis lung carcinoma; LNA, locked nucleic acid; LSD, the Fisher's least significant difference; NSCLC, non-small cell lung cancer; p-AKT, phosphorylation of protein kinase B; PBS, phosphate-buffered saline; PD-1, programmed cell death protein-1; p-mTOR, phosphorylation of mammalian target of rapamycin; Scr, scramble; SNORNA, small nucleolar RNA; TME, tumor microenvironment; Treg, regulatory T cells;.