Abstract
Worldwide, nations have struggled during the coronavirus disease 2019 (COVID‐19) pandemic. However, Latin America and the Caribbean faced an unmatched catastrophic toll. As of March 2022, the region has reported approximately 15% of cases and 28% of deaths worldwide. Considering the relatively late arrival of SARS‐CoV‐2, several factors in the region were determinants of the humanitarian crisis that ensued. Pandemic unpreparedness, fragile healthcare systems, forthright inequalities, and poor governmental support facilitated the spread of the virus throughout the region. Moreover, reliance on repurposed and ineffective drugs such as hydroxychloroquine and ivermectin—to treat or prevent COVID‐19—was publicised through misinformation and created a false sense of security and poor adherence to social distancing measures. While there were hopes that herd immunity could be achieved after the region's disastrous first peak, the emergence of the Gamma, Lambda, and Mu variants made this unattainable. This review explores how Latin America and the Caribbean fared during the first 2 years of the pandemic, and how, despite all the challenges, the region became a global leader in COVID‐19 vaccination, with 63% of its population fully vaccinated.
Keywords: SARS‐CoV‐2, COVID‐19, Latin America, vaccination, global health

Introduction
The coronavirus disease 2019 (COVID‐19) pandemic has not advanced similarly between countries. It has catastrophically affected some nations much more than others. Following the outbreak in Wuhan City in the Hubei Province of China in early 2020, a rapid international spread introduced the novel coronavirus, SARS‐CoV‐2, in virtually all territories around the globe [1]. In Latin America and the Caribbean (LAC), the first case was reported in Brazil on 26 February 2020, and the first death was announced shortly after in Argentina on 7 March [2, 3]. Relatively speaking, the virus was introduced late, giving the region valuable time to prepare based on strategies that were being used elsewhere. Nonetheless, its arrival was still catastrophic.
As of 2 March 2022, 65.4 million confirmed cases and 1.65 million deaths have been reported in LAC. With a total population of more than 652 million inhabitants (8% of the world population), these amount to approximately 15% of cases and 28% of deaths reported worldwide [4, 5] (Fig. 1). Prior to the Omicron wave, LAC was the epicentre of the SARS‐CoV‐2 pandemic with the highest proportions of cases and deaths among all other regions [6]. Amidst consecutive waves, LAC has not only faced a devastating tally but also crippling social and economic costs [7]. In this review, we aim to provide an overview of how this socioeconomically and politically complex region has succumbed to the magnitude and persistence of this public health crisis [8].
Fig. 1.
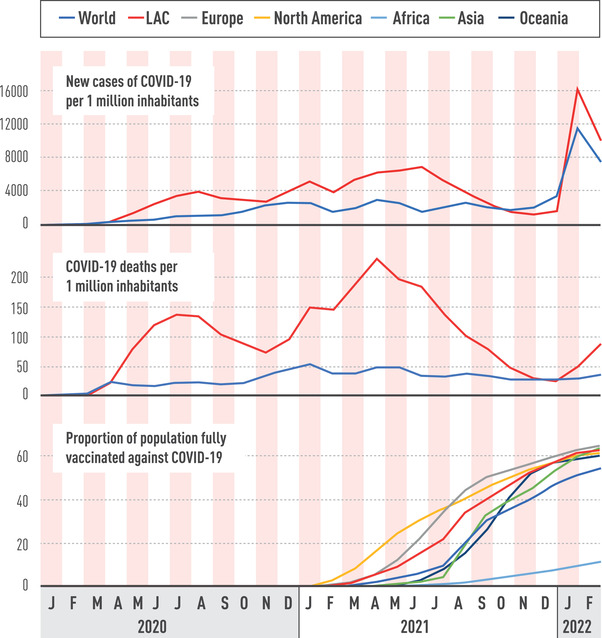
New cases, deaths, and proportion of population fully vaccinated in Latin America and the Caribbean. Source: ourworldindata.org/coronavirus.
Preparedness and initial response
The Global Health Security (GHS) Index assesses the national capability to address the threat of an international infectious disease agent [9]. In the months prior to the first reported cases of COVID‐19, all LAC countries had failed to meet the high‐score band (≥66.7/100) for pandemic preparedness [9]. Brazil, Argentina, Chile, and Mexico were ranked as the most prepared LAC countries with GHS Index scores ranging from 57.6 to 59.7 [9]. This transparency in preparedness might not be easily translated into practice, with some countries overperforming during the pandemic, as in the case of New Zealand, which ranked 35th in the GHS Index report [9]. Sadly, LAC was assessed fairly, with most countries underperforming during the pandemic. In retrospect, these assessments suffer discrepancies which suggest that other factors come into play, such as the effect of exceptional leadership in times of crises. Therefore, frequent re‐evaluation of the GHS Index should be considered [10].
According to the World Health Organisation (WHO), most LAC countries fail to reach the global average of 2.9 hospital beds per 1000 inhabitants, which underlines their chronic and severe underfunding [11]. In Latin America, Argentina rises above the global average with 4.9 hospital beds per 1000 inhabitants, and both Brazil and Chile have an estimated 2.1 hospital beds per 1000 inhabitants. On the other hand, Peru and Colombia have an even lower estimate of 1.6, with Ecuador close by with 1.5 hospital beds per 1000 inhabitants [11]. For countries in the Caribbean, this estimate is even lower, with Haiti having only 0.7 hospital beds per 1000 inhabitants [11]. The same can be said about Intensive Care Unit (ICU) beds since, before the pandemic, many LAC countries had ICU bed numbers that were below the bare minimum of 6 per 100,000 inhabitants [12]. Only Brazil and Argentina reported 26 and 19 ICU beds per 100,000 inhabitants, respectively [12, 13, 14]. In the first months of the pandemic, ICUs were prioritised, and countries were able to increase their capacity slightly [12]. A massive increase was seen in Mexico, whose ICU beds rose from 2 to 25 per 100,000 inhabitants [12]. Like other countries in the region, Mexico's population displays a high prevalence of obesity, diabetes, and hypertension, which places a large proportion of them at risk of severe COVID‐19 and, consequently, ICU bed requirements [15]. Underinvestment, however, has not only been visible in hospital resources but also in the shortage of qualified healthcare workers, which is one of the biggest problems the region has to face [16]. The Pan American Health Organisation (PAHO) has estimated that around 50,000 additional physicians and nurses are required for ICU needs to be met [17]. Having both adequate staff and hospital resources is essential to meeting the healthcare demand during this critical time [18].
Considering the unpreparedness of the region, immediate actions were necessary as the introduction of SARS‐CoV‐2 loomed. Governments’ responses to the pandemic have varied greatly. However, the initial measures were very similar in regard to magnitude and timing [13, 19]. Restrictions were set in place to evaluate and, if needed, isolate travellers arriving in the last days of February 2020. Nevertheless, by the second week of March, Argentina, Colombia, Chile, and Peru had enforced total lockdowns to prevent the spread of SARS‐CoV‐2, less than 10 days after each country detected its first COVID‐19 case [19]. Additional measures, including quarantines and curfews, were also implemented [13, 19]. These restrictions were extremely effective in reducing the number of cases and, for LAC countries with precarious healthcare systems, a necessity to avoid the collapse of hospitals [20]. Nonetheless, governments eventually needed to balance the prevention of disease transmission with the repercussions of the measures in the economy.
Socioeconomic impact and excess mortality
Apart from a health crisis, COVID‐19 has also led to a humanitarian crisis in the region (Fig. 2) [21]. A decades‐long backlog of public health underfunding was a determinant of the impact encountered during the pandemic. LAC invests an average of 7.9% (range = 3.56–11.19) of the gross domestic product (GDP) while Europe and North America have a current health expenditure of 9.8% and 16.4%, respectively [22]. In January 2021, the International Monetary Fund estimated a 7.4% economic contraction in the region, with several countries projecting GDP declines of over 10% [23]. Moreover, economic recovery was expected to be lower for the LAC region in comparison to the global economic growth for both 2021 (4.1% vs. 5.5%) and 2022 (3% vs. 4.9%) [23]. Social disparities have been exacerbated by this decline.
Fig. 2.
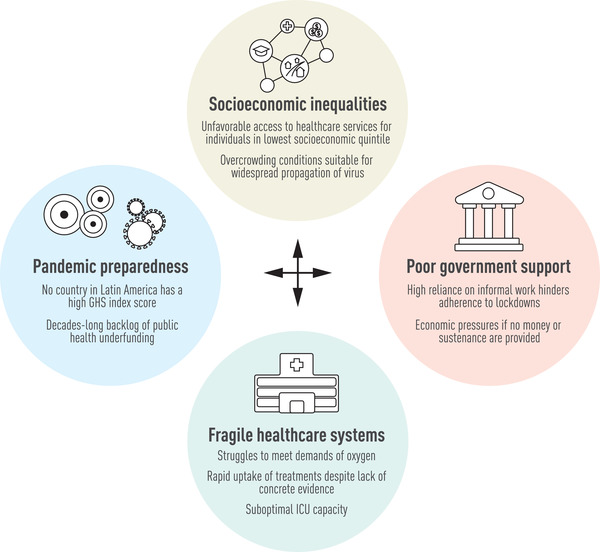
Interconnected determinants for the overwhelming death toll and crippling socioeconomic impact in Latin America and the Caribbean during the COVID‐19 pandemic.
The region as a whole reports an employment rate that is 11% lower than in pre‐pandemic times [24]. The extensive inequalities in LAC have had grave consequences for people of low socioeconomic status and those who relied on informal work for daily income [25]. Despite the early uptake of strict control measures, the existing social structure was not compatible with the governments’ pleas to stay at home and prevent transmission [13]. For some, understanding the risks involved with the community spread of SARS‐CoV‐2 led to increased willingness to follow social distancing measures [26]. Ironically, for many others, the decision to continue to work albeit exposed to a potentially deadly infection was the only way to ensure survival [27]. Although this has been the case for all countries, the strict containment policies have especially hurt the poorer regions of the world [28]. Studies suggest that household income was a determinant of the adherence to social distancing norms [26]; this was considered by most countries that opted to apply economic and social policies to support the vulnerable population [19, 29]. Measures that were not accompanied by government support in the form of money or sustenance crumbled from the economic pressures applied to households at risk [28]. For instance, urban slums were particularly struck as overcrowded conditions were suitable grounds for the widespread propagation of the virus [30, 31, 32]. Furthermore, a seroprevalence study at the peak of the first wave in Lima, Peru showed how lower socioeconomic status was linked to higher seroprevalence of SARS‐CoV‐2 infection [33]. Likewise, mortality rates were higher in deprived areas of Brazil, even in the early months of the pandemic [34]. These social inequities have been highlighted during the pandemic, as large gaps in access to healthcare have contributed to elevated out‐of‐pocket expenditure [21, 35, 36].
Despite the region's effort to mitigate the spread of the virus, the social standings ushered in a high death toll. The case‐fatality rate among LAC countries was averaged at 3.4%, with Mexico and Brazil reporting a lethality of 16.6% and 7.6%, respectively [31, 37]. Guayaquil, Ecuador's biggest city, was faced with an uncontrolled amount of deaths, where hundreds of COVID‐19 patients were left to die at home, with their bodies ending up on the streets [38]. Likewise, Ecuador's capital, Quito, experienced a gruesome sight of the pandemic as people turned to the use of cardboard coffins as a consequence of the surplus of abandoned corpses [39]. In addition, a survey revealed that seven in 10 Peruvians personally knew someone who died from COVID‐19 [40]. Nonetheless, testing capabilities were deficient in the early months, with an estimated rate of 63 tests per 100,000 inhabitants, the lowest rate worldwide [41]. Reporting deaths due to COVID‐19 proved to be a laborious task, for which many nations turned to evaluating all‐cause excess mortality as an effective alternative to track the pandemic [42]. Approximately 1.5–1.8 million excess deaths have been reported in the LAC region, with Peru currently reporting over 600 deaths per 100,000 inhabitants, the highest mortality per capita worldwide [43, 44]. For reference, the second‐highest mortality rate in the region is reported in Brazil, with over 290 deaths per 100,000 inhabitants [43]. Figure 3 provides a detailed overview of the mortality rates in the LAC countries as compared to the world average, showing how the virus has not stopped severely affecting the region throughout consecutive waves. Unfortunately, these numbers underline the deficiencies of the region's healthcare sector.
Fig. 3.
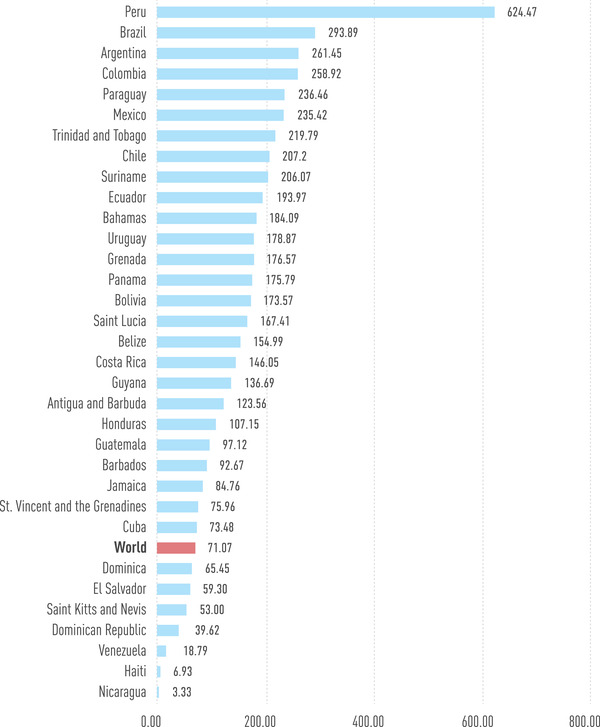
COVID‐19 mortality rates per 100,000 inhabitants in countries of Latin America and the Caribbean [Updated: 24 January 2022]. Source: worldometers.info/coronavirus/.
Healthcare systems vary greatly between LAC countries; however, most use a mixed system with both public and private sectors [13, 30]. While this could be seen as a means of extending access to healthcare services, it favours people in the highest income quintile compared to the lowest quintile [45]. Early measures were intended to flatten the curve and, among other reasons, ensure the continuation of the provision of healthcare. However, these fragmented and collapsed systems, with suboptimal ICU capacity, were charged with facing the pandemic head‐on [12, 13]. In most countries, hospitals were struggling to meet the demands for oxygen to treat COVID‐19 patients, which plays a vital role in patient survival. [15]. For cohorts in Peru and Mexico, hypoxemia was independently associated with elevated in‐hospital mortality of 49.6% and 30.1%, respectively [46, 47]. Brazil had to cope with providing over 340,000 cylinders (7 m3) of oxygen per day, with Argentina, Colombia, Mexico, and Peru trailing with approximately 100,000 cylinders each [48]. The failure to meet this necessity translated into patients’ relatives having to resort to a personal hunt for oxygen, which in many cases was met with an overwhelming amount of costs [49]. This regional oxygen shortage resulted in towering numbers of preventable deaths, for which the WHO‐led COVID‐19 Oxygen Emergency Taskforce was launched [50]. A case can be made for oxygen being as essential as electricity or water, as it is an integral resource in the management of medical conditions and its unavailability might be a death sentence for some.
The healthcare sector, however, is nothing without its staff [18]. During the last 2 years, healthcare workers have experienced insurmountable amounts of pressure and daily stressors that have severely affected their mental health [51]. This was especially the case in low‐ and middle‐income countries, where difficult triage decisions had to be made due to the deficiencies in healthcare [51]. In particular, 23.9% of healthcare professionals in LAC experienced post‐traumatic stress disorder symptoms, with Argentina and Chile scoring the highest amongst the region (26.4% and 29.8%, respectively) [52]. In addition, the fear that healthcare professionals might be carrying the virus led to them facing an enormous amount of discrimination, particularly in Colombia and Mexico [52]. Moreover, resilience has been tested constantly during the pandemic, with many falling ill. A meta‐analysis estimated that over 50% of healthcare personnel were reported to be infected with COVID‐19 just during the first 6 months of the pandemic [53]. Even worse, the WHO reported that approximately 115,500 healthcare professionals died globally as a result of COVID‐19 infection [54]. The system relies on its greatest asset, the healthcare workers. Therefore, not protecting them or overextending their reach to mend the system's deficiencies is not an option.
“Miracle” drugs and infodemia
In the early days of the pandemic, with the alarming exponential increase of hospitalisations and deaths, the entire world yearned for a panacea. With vaccine rollout ways down the line, any medication with anecdotal success or biological plausibility (in some cases, without the latter) was repurposed and used to treat COVID‐19 patients [55]. LAC was no stranger to the global craze over the unproven effects of hydroxychloroquine, azithromycin, or ivermectin [56]. The prospect of cheap, relatively safe, and widely available medications was too good of an offer for health services to ignore. These drugs were hastily incorporated into the national management guidelines, and in parallel randomised clinical trials were started to evaluate their efficacy [57]. Over 200 intervention studies from LAC were registered in the WHO International Clinical Trials Registry Platform during the first 6 months of the pandemic. The use of hydroxychloroquine, with or without azithromycin, was the most common type of intervention evaluated in the region, with 31 studies registered in the platform [57]. However, a significant number of these trials had a small sample size (less than 200 participants) and lacked a control group. Therefore, any beneficial findings would fail to be robust and be placed under scrutiny [57]. Interest in these drugs was seen not only among researchers, but trends from Google searches in Brazil, Mexico, and Peru showed a general interest in dexamethasone, hydroxychloroquine, and, in particular, ivermectin [58].
The antiparasitic ivermectin gained attention when reports claimed that it was a potent inhibitor of SARS‐CoV‐2 replication in vitro [59]. This glimmer of hope resulted in widely publicised fame. However, toxic concentrations were needed for a potentially beneficial effect to occur [60]. In spite of this, ivermectin was quickly incorporated into management guidelines for mild and severe cases of COVID‐19 in Peru and Bolivia, before any concrete evidence of efficacy from clinical trials had been found [61]. Actually, many clinical trials studying the use of ivermectin for COVID‐19 treatment have shown no effect over placebo [62]. One of the largest trials of ivermectin was found to have evidence of both plagiarism and discrepancies in the data set [63, 64]. Before being withdrawn, the paper was cited several times and was even included in a meta‐analysis that has since been retracted [65]. Later, the drug's use extended to preventive therapy, whose ultimate effect was the creation of a false sense of security and poor adherence to proven preventive measures [66]. It got so far that shortages of ivermectin, due to high and continued demand, led to the use of veterinary formulations of the drug instead [36]. One of the most important determinants of its popularity was the fact that this medication was easily obtained over‐the‐counter and was relatively cheap [66]. Dispensation of the drug was not controlled, allowing for non‐medical recommendations from friends, neighbours, public figures on TV, advertisements, and even forwarded messages on the phone [66]. The ivermectin frenzy even reached high‐income countries where vaccination campaigns were already further along than in LAC [67].
The rapid uptake of these drugs into national guidelines despite lack of concrete evidence was not met with an equally rapid removal when there was overwhelming evidence against their use. Worst of all, it was even harder to detach these drugs from the public who continued to rely on these “miracle drugs” that they could get without restrictions at any pharmacy. A reason for this includes the overwhelming amount of news connected to treatment solutions for COVID‐19, usually originating from social media platforms. Fake news started gaining political value within the region, tempting individuals to make false claims or reach for ineffective solutions to the pandemic [68]. Trends of increased COVID‐19 mortality in LAC have been linked to higher use and trust of social media as a means of obtaining health information [69]. A tracker of infodemia—an epidemic of false or misleading information—has observed a high Infodemic Risk Index (99.1%) in Peru, the highest in the LAC region [70]. This suggests that the population is very likely to encounter a social media post with potentially harmful information about COVID‐19, with a moderate chance of re‐sharing or commenting on this information [70]. Another country in the region with a high Infodemic Risk Index was Costa Rica (72.8%), which has reported over 140 deaths per 100,000 inhabitants [43, 70]. Countries at medium risk of infodemia included Haiti, Guatemala, Colombia, and Brazil [70]. While it is understandable for the public to be willing to try anything to avoid the virus, it is more important to recognise when collective beliefs are causing harm or interfering with public health measures that do work.
Herd immunity and variants
Early on, the herd immunity threshold was placed between 60% and 80% [71]. For some cities in LAC that were facing devastating first waves, the ill‐conceived prospect of having reached the threshold was a hopeful wish. In Manaus, Brazil, 76% of the population were found to be infected with SARS‐CoV‐2 by October 2020 [72]. Similarly, a high seroprevalence of 70% was found in Iquitos, Peru, another Amazonian city [73]. The recorded high seroprevalence of SARS‐CoV‐2 in these cities was striking compared to cities in Europe [74]. Some reports indicate the possibility of immunological cross‐reactivity with the dengue virus—which is prevalent in both Amazonian cities—explains the high seroprevalence encountered [75]. Nonetheless, they both presented a second peak, which suggests that the likelihood of herd immunity through infection might be unattainable [76]. Other studies were carried out in LAC, some of which also found high seroprevalence (Table 1). It is likely that waning immunity and/or immune evasion by new variants was responsible here [76].
Table 1.
SARS‐CoV‐2 seroprevalence studies conducted in Latin America and the Caribbean during the COVID‐19 pandemic
| Study | Country | Region/City | Date | Participants | Method | Seroprevalence (95% CI) |
|---|---|---|---|---|---|---|
| Figar et al. [112] | Argentina | Barrio Mugica, Buenos Aires | June 2020 | Individuals aged 14 or older (n = 873) | ELISA antibody test | 53.4% (52.8–54.1) |
| Rodeles et al. [113] | Argentina | Santa Fe | July–November 2020 | Adults aged 18 years or older (n = 3000) | ELISA antibody test | 8.83% |
| Armorim Filho et al. [114] | Brazil | Rio de Janeiro | April 2020 | Blood donors without COVID‐19 contact or symptoms 30 days before donation (n = 2857) | Immunochromatographic assay | 4.0% (3.3–4.7) |
| Silveira et al. [115] | Brazil | Rio Grande do Sul | April–May 2020 (three rounds of surveys) | All individuals (n = 13,111) | Lateral flow antibody test by finger prick blood sample | 0.0048% (0.006–0.174), 0.135% (0.049–0.293), and 0.222 (0.107–0.408) |
| Tess et al. [116] | Brazil | Sao Paulo | May 2020 | Adults 18 years or older (n = 463) | Chemiluminescent immunoassay by venous blood sample | 6.0% (3.9–8.3) |
| Hallal et al. [117] | Brazil | Various sentinel cities | May and June 2020 | Individuals ages 1 year or older (n = 25,025 and n = 31,165) | Lateral flow antibody test by finger prick blood sample | City‐level prevalence ranged from 0% to 25·4% in both surveys |
| De Souza Araujo et al. [118] | Brazil | Sergipe | July 2020 | Individuals of all ages (n = 5615) | Lateral flow antibody test by finger prick blood sample | 9.3% (8.5–10.1) |
| Pasqualotto et al. [119] | Brazil | Rio Grande do Sul | July 2020 | Military police force (n = 1592) | ELISA antibody test | 3.3% |
| Silva et al. [120] | Brazil | Maranhao | July–August 2020 | Individuals ages 1 year or older (n = 3289) | Electrochemiluminescence immunoassay | 40.4% (35.6–45.3) |
| Do Couto et al. [121] | Brazil | Sao Paulo | August 2020 | Persons experiencing homelessness (n = 203), and shelter workers (n = 87) | ELISA antibody test | 54.7% (47.8–61.5) in persons experiencing homelessness and 47.1% (36.6–57.6) in shelter workers |
| Lalwani et al. [122] | Brazil | Manaus | August–October 2020 | Adults aged 18 years or older (n = 3046) | ELISA antibody test | 29.1% |
| Cristelli et al. [123] | Brazil | Sao Paulo | September 2020 | Kidney transplant recipients (n = 416) | Chemiluminescent immunoassay by venous blood sample | 8.2% (5–11) |
| Miraglia et al. [124] | Brazil | Sao Paulo | September–December 2020 | Adults 18 years or older (n = 272) | Immunoassay from venous blood sample | 43.8% (37.7–50.0) |
| Cavalcante Pinto et al. [125] | Brazil | Fortaleza | November–December 2020 | Individuals of all ages (n = 5615) | Immunochromatographic assay by finger prick blood sample | Children = 25.3%; adolescents = 29.2%; adults = 20.9% |
| Borges et al. [126] | Brazil | Sergipe | January 2021 | Firefighters (n = 123) | Immunofluorescence assay by venous blood sample | 45.5% (36.5–54.7) |
| Malagon‐Rojas et al. [127] | Colombia | Bogota | June–September 2020 | Airport workers from 18 to 60 years of age (n = 212) | Chemiluminescent immunoassay by venous blood sample | 23.58% (18.37–29.74) |
| Mattar et al. [128] | Colombia | Monteria | August 2020 | Individuals of all ages (n = 1368) | ELISA antibody test | 55.3% (52.5–57.8) |
| Colmenares‐Mejia et al. [129] | Colombia | Bucaramanga Metropolitan Area | September–December 2020 | Occupational groups aged 18 years or older (n = 7045) | Chemiluminescent immunoassay by venous blood sample | 19.5% (18.6–20.4) |
| Del Brutto et al. [130] | Ecuador | Atahualpa Canton | May 2020 | Adults 40 years or older in rural areas (n = 673) | Lateral flow antibody test by finger prick blood sample | 45% |
| Acurio‐Paez et al. [131] | Ecuador | Cuenca | August–November 2020 | All individuals (n = 2457) | ELISA antibody test | 13.2% (12–14.6) |
| Flamand et al. [132] | French Guiana | Various municipalities | July 2020 | All individuals (n = 480) | ELISA antibody test | 15.4% (9.3–24.4) |
| Muñoz‐Medina et al. [133] | Mexico | Nation‐wide | February–December 2020 | All individuals without reported fever in the previous 2 weeks (n = 24,273) | Chemiluminescent immunoassay by venous blood sample | 3.5% in February and 33.5% in December. |
| Diaz‐Salazar et al. [134] | Mexico | Guadalupe, Nuevo León | July 2020 | Government employees (n = 3268) | Chemiluminescent immunoassay by venous blood sample | 5.9% (5.1–6.7) |
| Cruz‐Arenas et al. [135] | Mexico | Mexico City | August–September 2020 | Healthcare workers in a non‐COVID hospital (n = 299) | Immunochromatographic assay by venous blood sample | 11.0% |
| Rodriguez‐Vidales et al. [136] | Mexico | Nuevo Leon | August–November 2020 | Adults with no suggestive symptoms or prior diagnosis of COVID‐19 (n = 4495) | Chemiluminescent immunoassay by venous blood sample | 27.1% (25.8–28.4) |
| Diaz‐Velez et al. [137] | Peru | Lambayeque | June–July 2020 | Individuals aged 9 years or older (n = 2010) | Immunochromatographic assay by finger prick blood sample | 29.5% (27.6–31.5) |
| Reyes‐Vega et al. [33] | Peru | Lima Metropolitan Area | June–July 2020 | Individuals of all ages (n = 3212) | Immunochromatographic assay by finger prick blood sample | 20.8% (17.2–23.5) |
| Álvarez‐Antonio et al. [73] | Peru | Iquitos | July and August 2020 | Individuals of all ages (n = 716) | Immunochromatographic assay by finger prick blood sample | 70% (67–73) at baseline and 66% (62–70) at 1 month follow‐up |
| Huamaní et al. [138] | Peru | Cusco | September 2020 | All individuals aged 18 or older (n = 1924) | Chemiluminescent immunoassay by venous blood sample | 38.8% (33.4–44.9) |
| Moreira‐Soto et al. [139] | Peru | San Martin | March 2021 | All individuals aged 5 years or older (n = 563) | Chemiluminescent immunoassay by venous blood sample and confirmation by SARS‐CoV‐2 surrogate virus neutralisation test (sVNT) | 59.0% (55–63) |
The rise in new cases and the collective start of the second wave in LAC were fuelled by the emergence of the P.1 variant, officially named Gamma [77]. This lineage acquired 17 mutations of which three conferred changes in the spike protein leading to increased binding to the human ACE2 receptor [54]. Classified as a variant of concern, there was evidence of an increase in transmissibility (1.4–2.2 times) and of immune evasion conferred by past infection with non‐Gamma lineages of SARS‐CoV‐2 [77]. Using molecular clock phylogenetics, it was estimated that the Gamma variant appeared in November 2020 in Manaus, which was experiencing the lowest levels of daily new confirmed cases since the beginning of the pandemic [77]. The emergence of the Gamma variant explains the increase of cases in the region [78]. The wide propagation of this variant was also linked to a decrease in preventive measures which were dependent on the decisions of each state and municipality. A stark contrast was seen in the number of reported cases during this second wave between Amazonas and Pará, its neighbouring state [79]. Both states had presented a similar number of cases during their first waves from May to November 2020, but had then differed as cases in Amazonas soared [79]. As a result of the propagation of the Gamma variant, Brazil was reporting a high daily death toll, surpassing 300,000 confirmed total deaths from COVID‐19 [80]. At present, Brazil has one of the highest total COVID‐19 deaths, second only to the United States [43].
LAC also faced two variants of interest: Lambda (C.37 lineage) and Mu (B.1.621 lineage), with the earliest documented samples from December 2020 in Peru and January 2021 in Colombia [81]. In the case of Lambda, it quickly became the predominant variant in Peru in early March 2021 and, as with Gamma in Brazil, it fuelled a harsher second wave [82]. Beyond Peru, the variant was also found in a high proportion of cases in Chile and Argentina [82]. Similarly, in Colombia, Mu was the main determinant of the start of the third wave in March 2021, which rose above prior surges and exhibited a two‐stage peak‐within‐a‐peak [83]. Mu's transmissibility in Colombia may have been aided by the easing of restrictions to stimulate the economy [83]. The discovery of these variants should bring praise to the genomic sequencing capacity of the region, in spite of its shortcomings. Needless to say, LAC was also confronted with other variants of concern. In July 2021, Mexico had the highest number of COVID‐19 cases due to the Delta (B.1.617.2) variant [84]. Although it spread, the aforementioned “local” variants of interest were predominantly driving the new waves of 2021. At present, the Omicron (B.1.1.529) variant is sweeping through the nations, producing infection rates at all‐time pandemic highs [85]. Thankfully, an equal rise in hospitalisations or deaths is not evident.
Vaccination
After a year of struggles and enforced social distancing measures, vaccine rollout started, albeit at a slow pace, by virtue of the COVID‐19 Vaccines Global Access (COVAX) project and negotiations with pharmaceutical companies [86]. In early March 2021, Colombia was the first country in LAC to receive a delivery of the Pfizer‐BioNTech vaccine through the COVAX program [87]. Nonetheless, it soon became apparent that distribution was not equitable. Only around 0.1% of the available vaccines worldwide were shipped to low‐income regions, which goes against COVAX's promise of fair global access to vaccines [88, 89]. Consequently, in the first semester of 2021, vaccine coverage against COVID‐19 in LAC fell short compared to coverage in Europe and North America [86]. With many countries being far from the reach of the PAHO's recommended vaccination coverage, LAC still managed to fully vaccinate (two doses) 63% of its population, the highest percentage for any region in the world to date [87]. Uruguay and Chile have vaccinated a remarkable amount of their citizens, earning their place in the top five countries worldwide [90]. LAC's vaccine triumph was a pleasant surprise considering the region's rough and painful start. Nonetheless, previous insights into barriers of vaccination suggest there is wide variability across and within countries that could lead to pockets of the unprotected population [91].
Challenges of mass vaccination have arisen and can be classified into accessibility and individual factors, including vaccine hesitancy [91]. The former poses difficulties in the delivery of mRNA vaccines, such as Pfizer/BioNTech or Moderna, whose temperature storage requirements limit their use outside of big cities [92]. For the population residing deep in the Amazon or high in the Andes, a long journey would need to be taken to reach a vaccination centre with appropriate minus 80‐degree centigrade storage, and this would need to be repeated for the second and third doses. Vaccine hesitancy, on the other hand, leads to a bigger problem, as many people are worried about the speed at which the vaccines were developed. A Chilean study in late 2020 reported that 28% of respondents were indecisive about getting vaccinated and 23% refused to get the doses overall. Of note, the questions were not referring to a specific COVID‐19 vaccine [93]. Furthermore, some LAC citizens have shown distrust in the vaccines on offer, with high scepticism over the efficacy and safety of China's Sinovac and Russia's Sputnik [94]. Fortunately, hesitancy is low compared to other regions, particularly Europe [94, 95].
Approximately 4.9 million Venezuelan migrants reside in LAC [96]. During the pandemic, they have been at higher risk of serious illness due to COVID‐19 and barriers in accessing health services [97]. Vaccination policies of most of the countries hosting the majority of Venezuelan migrants mentioned access to “migrants” in their vaccination plans. However, only Colombia and Ecuador explicitly named “Venezuelan migrants” [86, 98]. Neither Brazil nor Chile had any mention of migrants in their vaccination plans [86, 98]. Sadly, inclusion in policies did not translate into effortless access to the COVID‐19 vaccine, as migrants were required to navigate through bureaucracy and even face discrimination [89, 98]. Similarly, the indigenous populations in LAC have had struggles with vaccination, mainly due to poor access to immunisation services [99, 100]. High seroprevalence and lethality due to SARS‐CoV‐2 has been found in this vulnerable population [101]. Governments have been called to consider the difficult logistical challenges with the provision of vaccines in remote areas, like the Amazon jungle [100]. Another barrier is the lack of trust these communities have towards government health services due to prior neglect. Strengthening the relationship between them is paramount in order to narrow the gap [99].
The region learned early on that vaccination was not an immediate return to normality, even when administered at a rapid pace. Despite the fastest per‐capita COVID‐19 vaccination campaign, Chile was forced into a new strict lockdown in March 2021 amidst a soaring rise in cases [102]. While some blame was attributed to the Gamma or Lambda variant, responsibility can also be placed on the premature and sudden reopening of the country and the easing of restrictions [103]. Nevertheless, LAC can now see the positive effects that vaccines are having on the population's well‐being. Several vaccine effectiveness studies were conducted in LAC, which provided reassuring results in a time when just a fraction of the population, particularly the elderly, had received their doses [Table 2]. Now that the coverage is much greater, the effects of the vaccination programs are observable with case‐fatality among LAC countries dropping significantly. For instance, Mexico and Brazil report a lethality of 6.5% (from 16.6%) and 2.6% (from 7.6%), respectively [104]. The recent surge due to the Omicron variant is further evidence that cases decouple from hospitalisations and deaths [104]. While this might also indicate that the current wave is milder, it has the potential to cause severe disease in populations who are yet to be vaccinated or to once again overwhelm healthcare services [105].
Table 2.
Vaccine effectiveness studies in Latin America and the Caribbean during the COVID‐19 pandemic
| Study | Country | Vaccine | Doses and schedule | Participants | Effectiveness (95% CI) | Other conclusions |
|---|---|---|---|---|---|---|
| González et al. [140] | Argentina | Gam‐COVID‐Vac (Sputnik V) | One dose, first component | Individuals aged 60–79 years (n = 40,387) | 78.6% (74.8–81.7) for preventing laboratory‐confirmed infections | Prevention of hospitalisation (87.6%; 95% CI: 74.8–81.7), and death (84.8%; 95% CI: 75.0–90.7) |
| Hitchings et al. [141] | Brazil | AZD1222 (Oxford‐AstraZeneca) | Two doses (0 and 21 days) | Adults aged 60 years or older (n = 137,744) | 77.9% (69.2–84.2) for prevention of symptomatic SARS‐CoV‐2 infection | Prevention of hospitalisation (87.6%; 95% CI: 78.2–92.9), of ICU admission (89.9%; 95% CI: 70.9–96.5), invasive mechanical ventilation (96.5%; 95% CI 81.7–99.3), and death (93.6%; 95% CI 81.9–97.7) in the fully immunised group. |
| Alencar et al. [142] | Brazil | CoronaVac (Sinovac Life Sciences) and AZD1222 (Oxford‐AstraZeneca) | Two doses | Elderly people aged 75 years or older (n = 313,328) | Protection ratio of 132.67 (109.88–160.18) against COVID‐19‐related death with two doses. | Protection ratio of 19.31 (18.20–20.48) against COVID‐19‐related death with one dose. |
| Hitchings et al. [143] | Brazil | CoronaVac (Sinovac Life Sciences) |
Two doses (0 and 14 days) (n = 53,176) |
Healthcare workers, aged 18 years or older | 37.1% (53.3–74.2) for prevention of symptomatic SARS‐CoV‐2 infection | At least one dose was associated with an effectiveness of 49.4% (95% CI 13.2–71.9) for prevention of symptomatic SARS‐Cov‐2 infection |
| Ranzani et al. [144] | Brazil | CoronaVac (Sinovac Life Sciences) | Two doses (0 and 14 days) | Individuals aged > 70 years (n = 43,774) | 46.8% (38.7–53.8) for preventing symptomatic COVID‐19 | Effectiveness against hospital admissions was 55.5% (95% CI 46.5–62.9) and against death was 61.2% (95% CI 48.9–70.5) |
| Jara et al. [145] | Chile | CoronaVac (Sinovac Life Sciences) | Two doses (0 and 14 days) | Participants 16 years of age or older (n = 10,187,720) | 65.9% (65.2–66.6) for the prevention of COVID‐19 (n = 4,173,574) | Prevention of hospitalisation (87.5%; 95% CI: 86.7–88.2), of ICU admission (90.3%; 95% CI: 89.1–91.4) and death (86.3%; 95% CI 84.5–87.9) in the fully immunised group. |
| Murillo‐Zamora et al. [146] | Mexico | BNT162b2 (Pfizer‐BioNTech) | Two doses (0 and 21 days) | Healthcare workers aged 18 years and above with laboratory‐confirmed COVID‐19 (n = 312) | Vaccine effectiveness was 100% against severe illness after one or two doses of the vaccine | ‐ |
| Silvia‐Valencia et al. [147] | Peru | BBIPB‐CorV (Sinopharm) | Two doses (0 and 21 days) | Healthcare workers aged 18 years and above (n = 606,772) | 50.4% (49.0–52.0) for prevention of SARS‐CoV‐2 infection after two doses | 94.0% (91.0–96.0) for prevention of COVID‐19 related‐death after two doses |
Final remarks
While the battle continues, LAC is compelled to address additional problems that arose as a consequence of the pandemic. As ICU and emergency services were presented with an influx of cases, it remains to be seen how the healthcare system will fare against the population suffering disability from the long‐term effects of COVID‐19 [106]. Furthermore, children in the region have missed more school than anyone else worldwide. The effects of this disruption in education may continue to be felt for years to come [107]. Sadly, another secondary impact of the pandemic has been the distressing number of children affected by orphanhood, with an estimate of more than 1 in 1000 children in several countries in LAC [108]. Moreover, stay‐at‐home measures contributed to the rise in domestic violence and sexual abuse. Calls to help hotlines increased massively in Colombia—which received 127% more reports than usual—and in Argentina, with sexual violence rising more than two‐thirds [109]. Whilst the region has managed to show signs of economic growth, complete rebound is not expected to occur in the following years, imposing added misfortune to the citizens of LAC [110]. Despite the brutal reality and shortcomings, the pandemic has not drastically altered trust in the healthcare system [111]. The vaccination campaigns and adherence to public health guidelines have started to alleviate the pandemic, with the LAC region becoming the global leader in vaccination. The population is finally able to steadily, and safely, resume its social interactions and start down the road to recovery.
Conflict of interest
The authors declare no conflict of interest.
Author Contributions
Alvaro Schwalb: Investigation; Methodology; Writing – original draft; Writing – review & editing. Eleonora Armyra: Investigation; Writing – original draft; Writing – review & editing. Melissa Méndez‐Aranda: Investigation; Writing – review & editing. César Ugarte‐Gil: Methodology; Writing – original draft; Writing – final review.
Schwalb A, Armyra E, Méndez‐Aranda M, Ugarte‐Gil C. COVID‐19 in Latin America and the Caribbean: Two years of the pandemic. J Intern Med. 2022;1–19.
References
- 1. Phelan AL, Katz R, Gostin LO. The Novel Coronavirus Originating in Wuhan, China: Challenges for Global Health Governance. JAMA. 2020;323:709–10. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . COVID‐19: Situation Report 38. WHO, Geneva, 2020. [Google Scholar]
- 3. World Health Organization . COVID‐19: Situation Report 48. WHO: Geneva, 2020. [Google Scholar]
- 4. The World Bank . Population, total ‐ Latin America & Caribbean. The World Bank. [Google Scholar]
- 5. Reuters . Latin America and the Caribbean: the latest coronavirus counts, charts and maps. Reuters ‐ COVID‐19 Tracker. 2020. [Google Scholar]
- 6. Pearson S, Magalhaes L. South America Is Now Covid‐19 Hot Spot, With Eight Times the World's Death Rate. WSJ Online. 2021. [Google Scholar]
- 7. Gonzalez E, Harrison C, Hopkins K, Horwitz L, Nagovitch P, Sonneland HK, et al. The Coronavirus in Latin America. Americas Society/Council of the Americas 2021.
- 8. Callejas D, Echevarría JM, Carrero Y, Rodríguez‐Morales AJ, Moreira R. The SARS‐CoV‐2 pandemic in Latin America: the need for multidisciplinary approaches. Curr Trop Med Rep. 2020: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cameron EE, Nuzzo JB, Bell JA. Global Health Security Index: Building Collective Action and Accountability. Nuclear Threat Initiative & Johns Hopkins Center for Health Security, 2019. [Google Scholar]
- 10. Abbey EJ, Khalifa BAA, Oduwole MO, Ayeh SK, Nudotor RD, Salia EL, et al. The Global Health Security Index is not predictive of coronavirus pandemic responses among Organization for Economic Cooperation and Development countries. PLoS One. 2020;15:e0239398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization . The Global Health Observatory. WHO. [Google Scholar]
- 12. Torres F. Latinoamérica en Cuidados Intensivos. Salud con Lupa. 2020. [Google Scholar]
- 13. Garcia PJ, Alarcón A, Bayer A, Buss P, Guerra G, Ribeiro H, et al. COVID‐19 response in Latin America. Am J Trop Med Hyg. 2020;103:1765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sen‐Crowe B, Sutherland M, McKenney M, Elkbuli A. A closer look into global hospital beds capacity and resource shortages during the COVID‐19 pandemic. J Surg Res. 2021;260:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. The Economist . Hospitals are running out of oxygen to treat COVID‐19 patients. The Economist. 2021. [Google Scholar]
- 16. Bastías G, Poblete F. Improving the performance of hospitals and the health system in Latin America and the Caribbean. Lancet Glob Health. 2021;9:e1045–6. [DOI] [PubMed] [Google Scholar]
- 17. Epstein D, Nusser N, Oliel S, Baldwin A, Peimbert‐Rappaport N. PAHO steps up assistance to help countries cope with shortages of oxygen and health workers. PAHO. 2021. [Google Scholar]
- 18. Kahn JM, Rubenfeld GD. The myth of the workforce crisis. Why the United States does not need more intensivist physicians. Am J Respir Crit Care Med. 2015;191:128–34. [DOI] [PubMed] [Google Scholar]
- 19. Martinez‐Valle A. Public health matters: why is Latin America struggling in addressing the pandemic? J Public Health Policy. 2021;42:27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alfano V, Ercolano S. The efficacy of lockdown against COVID‐19: a cross‐country panel analysis. Appl Health Econ Health Policy. 2020;18:509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. The Lancet . COVID‐19 in Latin America: a humanitarian crisis. Lancet. 2020;396:1463. [DOI] [PubMed] [Google Scholar]
- 22. The World Bank . Current health expenditure (% of GDP) ‐ Latin America & Caribbean, European Union, North America. The World Bank. [Google Scholar]
- 23. International Monetary Fund . World Economic Outlook Update. IMF, 2021. [Google Scholar]
- 24. The World Bank . An Uneven Recovery: the Impact of COVID‐19 on Latin America and the Caribbean. The World Bank; 2021. [Google Scholar]
- 25. Bárcena Ibarra A, Byanyima W. Latin America is the world's most unequal region. Here's how to fix it. World Economic Forum. 2016. [Google Scholar]
- 26. Alicea‐Planas J, Trudeau JM, Vásquez Mazariegos WF. COVID‐19 Risk Perceptions and Social Distancing Practice in Latin America. Hisp Health Care Int. 2021: 1540415320985141. [DOI] [PubMed] [Google Scholar]
- 27. Chriscaden K. Impact of COVID‐19 on people's livelihoods, their health and our food systems. WHO 2020. [Google Scholar]
- 28. Bargain O, Aminjonov U. Poverty and COVID‐19 in Africa and Latin America. World Dev. 2021;142:105422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Organisation for Economic Co‐operation and Development . COVID‐19 in Latin America and the Caribbean: An overview of government responses to the crisis. OECD, 2020. [Google Scholar]
- 30. Barreto SM, Miranda JJ, Figueroa JP, Schmidt MI, Munoz S, Kuri‐Morales PP, et al. Epidemiology in Latin America and the Caribbean: current situation and challenges. Int J Epidemiol. 2012;41:557–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ashktorab H, Pizuomo A, González NAF, Villagrana EDC, Herrera‐Solís ME, Cardenas G, et al. A comprehensive analysis of COVID‐19 impact in Latin America. Res Sq. 2021. [Google Scholar]
- 32. Rader B, Scarpino SV, Nande A, Hill AL, Adlam B, Reiner RC, et al. Crowding and the shape of COVID‐19 epidemics. Nat Med. 2020;26:1829–34. [DOI] [PubMed] [Google Scholar]
- 33. Reyes‐Vega MF, Soto‐Cabezas MG, Cárdenas F, Martel KS, Valle A, Valverde J, et al. SARS‐CoV‐2 prevalence associated to low socioeconomic status and overcrowding in an LMIC megacity: a population‐based seroepidemiological survey in Lima, Peru. EClinicalMedicine. 2021;34:100801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Silva J, Ribeiro‐Alves M. Social inequalities and the pandemic of COVID‐19: the case of Rio de Janeiro. J Epidemiol Community Health. 2021;75:975–9 [DOI] [PubMed] [Google Scholar]
- 35. Ribeiro KB, Ribeiro AF, de Sousa Mascena Veras MA, de Castro MC. Social inequalities and COVID‐19 mortality in the city of São Paulo, Brazil. Int J Epidemiol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bilal U, Alfaro T, Vives A. COVID‐19 and the worsening of health inequities in Santiago, Chile. Int J Epidemiol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sonneland HK. Chart: COVID‐19 Mortality Rates in Latin America and the Caribbean. Americas Society/Council of the Americas. 2020. [Google Scholar]
- 38. Moncada B. Coronavirus: “When coffins lined the streets of my hometown.” BBC. https://www.bbc.co.uk/news/av/world‐latin‐america‐53451703. Published July 19, 2020. Accessed January 25, 2022. [Google Scholar]
- 39. Cabrera JML, Kurmanaev A. Ecuador's Death Toll During Outbreak Is Among the Worst in the World. The New York Times. https://www.nytimes.com/2020/04/23/world/americas/ecuador‐deaths‐coronavirus.html. Published April 23, 2020. Accessed January 25, 2022. [Google Scholar]
- 40. Ipsos . Encuesta Nacional Urbana Agosto 2020 ‐ Actitudes hacia el COVID‐19. Ipsos, 2020. [Google Scholar]
- 41. Lima EEC, Vilela EA, Peralta A, Rocha M, Queiroz BL, Gonzaga MR, et al. Investigating regional excess mortality during 2020 COVID‐19 pandemic in selected Latin American countries. Genus. 2021;77:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. The Economist . Tracking covid‐19 excess deaths across countries. The Economist. 2021. [Google Scholar]
- 43. Worldometer . COVID Live ‐ Coronavirus Statistics. Worldometer. [Google Scholar]
- 44. The Economist . There have been 7m‐13m excess deaths worldwide during the pandemic. The Economist. https://www.economist.com/briefing/2021/05/15/there‐have‐been‐7m‐13m‐excess‐deaths‐worldwide‐during‐the‐pandemic. Published May 13, 2021. Accessed January 30, 2022. [Google Scholar]
- 45. Perel P, Casas JP, Ortiz Z, Miranda JJ. Noncommunicable diseases and injuries in Latin America and the Caribbean: time for action. PLoS Med. 2006;3:e344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mejía F, Medina C, Cornejo E, Morello E, Vásquez S, Alave J, et al. Oxygen saturation as a predictor of mortality in hospitalized adult patients with COVID‐19 in a public hospital in Lima, Peru. PLoS One. 2020;15:e0244171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Olivas‐Martínez A, Cárdenas‐Fragoso JL, Jiménez JV, Lozano‐Cruz OA, Ortiz‐Brizuela E, Tovar‐Méndez VH, et al. In‐hospital mortality from severe COVID‐19 in a tertiary care center in Mexico City; causes of death, risk factors and the impact of hospital saturation. PLoS One. 2021;16:e0245772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Market Dynamics . COVID‐19 Oxygen Needs Tracker. Path. [Google Scholar]
- 49. Uchoa P. Coronavirus: What's behind Latin America's oxygen shortages? BBC. https://www.bbc.co.uk/news/world‐latin‐america‐55829424. Published January 30 , 2021. Accessed March 22, 2021. [Google Scholar]
- 50. Verhoosel H, Baker C. COVID‐19 oxygen emergency impacting more than half a million people in low‐ and middle‐income countries every day, as demand surges. WHO. 2021. [Google Scholar]
- 51. Mehta S, Machado F, Kwizera A, Papazian L, Moss M, Azoulay É, et al. COVID‐19: a heavy toll on health‐care workers. Lancet Respir Med. 2021;9:226–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Abeldaño Zuñiga RA, Juanillo‐Maluenda H, Sánchez‐Bandala MA, Burgos GV, Müller SA, Rodríguez López JR. Mental health burden of the COVID‐19 pandemic in healthcare workers in four Latin American countries. Inquiry. 2021;58:469580211061059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gholami M, Fawad I, Shadan S, Rowaiee R, Ghanem H, Hassan Khamis A, et al. COVID‐19 and healthcare workers: a systematic review and meta‐analysis. Int J Infect Dis. 2021;104:335–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. World Health Organization . The impact of COVID‐19 on health and care workers: a closer look at deaths. WHO, 2021. [Google Scholar]
- 55. Cimerman S, Chebabo A, Cunha CA da, Rodríguez‐Morales AJ. One year after the arrival of COVID‐19 in Latin America: what have we learned in Brazil and other countries? Braz J Infect Dis. 2021;25:101571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schwalb A, Seas C. The COVID‐19 Pandemic in Peru: What Went Wrong? Am J Trop Med Hyg. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Carracedo S, Palmero A, Neil M, Hasan‐Granier A, Saenz C, Reveiz L. [The landscape of COVID‐19 clinical trials in Latin America and the Caribbean: assessment and challenges]. Rev Panam Salud Publica. 2021;45:e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chire Saire JE, Lemus‐Martin R. Analysis of internet trends related to medications for COVID‐19 in ten countries with the highest number of cases. bioRxiv. 2020. [Google Scholar]
- 59. Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA‐approved drug ivermectin inhibits the replication of SARS‐CoV‐2 in vitro. Antiviral Res. 2020;178:104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chaccour C, Hammann F, Ramón‐García S, Rabinovich NR. Ivermectin and COVID‐19: keeping rigor in times of urgency. Am J Trop Med Hyg. 2020;102:1156–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mega ER. Latin America's embrace of an unproven COVID treatment is hindering drug trials. Nature. 2020;586:481–2. [DOI] [PubMed] [Google Scholar]
- 62. Popp M, Stegemann M, Metzendorf M‐I, Gould S, Kranke P, Meybohm P, et al. Ivermectin for preventing and treating COVID‐19. Cochrane Database Syst Rev. 2021: 7:CD015017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Reardon S. Flawed ivermectin preprint highlights challenges of COVID drug studies. Nature. 2021;596:173–4. [DOI] [PubMed] [Google Scholar]
- 64. Meyerowitz‐Katz G. Does Ivermectin work for Covid‐19? Medium. 2021. [Google Scholar]
- 65. Hill A, Garratt A, Levi J, Falconer J, Ellis L, McCann K, et al. Erratum: Expression of Concern: “Meta‐analysis of Randomized Trials of Ivermectin to Treat SARS‐CoV‐2 Infection.” Open Forum Infect Dis. 2021;8:ofab394. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66. Molento MB. COVID‐19 and the rush for self‐medication and self‐dosing with ivermectin: a word of caution. One Health. 2020;10:100148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Food and Drug Administration . Why You Should Not Use Ivermectin to Treat or Prevent COVID‐19. FDA, 2021. [Google Scholar]
- 68. Ceron W, Gruszynski Sanseverino G, de‐Lima‐Santos M‐F, Quiles MG. COVID‐19 fake news diffusion across Latin America. Soc Netw Anal Min. 2021;11:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nieves‐Cuervo GM, Manrique‐Hernández EF, Robledo‐Colonia AF, Grillo AEK. Infodemic: fake news and COVID‐19 mortality trends in six Latin American countries. Rev Panam Salud Publica. 2021;45:e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fondazione Bruno Kessler . COVID‐19 Infodemics Observatory. covid19obs.
- 71. Gomes MGM, Ferreira MU, Corder RM, King JG, Souto‐Maior C, Penha‐Gonçalves C, et al. Individual variation in susceptibility or exposure to SARS‐CoV‐2 lowers the herd immunity threshold. medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Buss LF, Prete CA, Abrahim CMM, Mendrone A, Salomon T, de Almeida‐Neto C, et al. Three‐quarters attack rate of SARS‐CoV‐2 in the Brazilian Amazon during a largely unmitigated epidemic. Science. 2021;371:288–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Álvarez‐Antonio C, Meza‐Sánchez G, Calampa C, Casanova W, Carey C, Alava F, et al. Seroprevalence of anti‐SARS‐CoV‐2 antibodies in Iquitos, Peru in July and August, 2020: a population‐based study. Lancet Glob Health. 2021;9:e925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Grant R, Dub T, Andrianou X, Nohynek H, Wilder‐Smith A, Pezzotti P, et al. SARS‐CoV‐2 population‐based seroprevalence studies in Europe: a scoping review. BMJ Open. 2021;11:e045425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nicolelis MAL, Raimundo RLG, Peixoto PS, de Andreazzi CS. How super‐spreader cities, highways, hospital bed availability, and dengue fever influenced the COVID‐19 epidemic in Brazil. bioRxiv. 2020. [Google Scholar]
- 76. Taylor L. Covid‐19: Is Manaus the final nail in the coffin for natural herd immunity? BMJ. 2021;372:n394. [DOI] [PubMed] [Google Scholar]
- 77. Faria NR, Mellan TA, Whittaker C, Claro IM, Candido D da S, Mishra S, et al. Genomics and epidemiology of the P.1 SARS‐CoV‐2 lineage in Manaus, Brazil. Science. 2021;372:815–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Demoliner M, Silva MS da, Gularte JS, Hansen AW, de Almeida PR, Weber MN, et al. Predominance of SARS‐CoV‐2 P.1 (Gamma) lineage inducing the recent COVID‐19 wave in southern Brazil and the finding of an additional S: D614A mutation. Infect Genet Evol. 2021;96:105134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Emmerich FG. Comparisons between the neighboring states of Amazonas and Pará in Brazil in the Second Wave of COVID‐19 outbreak and a possible role of early ambulatory treatment. Int J Environ Res Public Health. 2021;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pearson S, Dube R. Covid‐19 variant in Brazil overwhelms local hospitals, hits younger patients. WSJ Online. 2021. [Google Scholar]
- 81. World Health Organization . Tracking SARS‐CoV‐2 variants. WHO. [PubMed] [Google Scholar]
- 82. Romero PE, Dávila‐Barclay A, Salvatierra G, González L, Cuicapuza D, Solis L, et al. The emergence of Sars‐CoV‐2 variant Lambda (C.37) in South America. Microbiol Spectr. 2021;9:e0078921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Schernhammer E, Weitzer J, Laubichler MD, Birmann BM, Bertau M, Zenk L, et al. Complex correlates of Colombia's COVID‐19 surge. Lancet Reg Health Am. 2021;3:100072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Harrison C. Explainer: Covid variants in Latin America. Americas Society/Council of the Americas. 2021. [Google Scholar]
- 85. Harrison C. How Latin America is faring against Omicron. Americas Society/Council of the Americas. 2022. [Google Scholar]
- 86. Marcela Vélez C. COVID‐19 y vacunación en América Latina y el Caribe: desafíos, necesidades y oportunidades. UNESCO, 2021. [Google Scholar]
- 87. Harrison C, Horwitz L, Zissis C. Timeline: tracking Latin America's road to vaccination. Americas Society/Council of the Americas. 2022. [Google Scholar]
- 88. Acharya KP, Ghimire TR, Subramanya SH. Access to and equitable distribution of COVID‐19 vaccine in low‐income countries. NPJ Vaccines. 2021;6:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zard M, Lau LS, Bowser DM, Fouad FM, Lucumí DI, Samari G, et al. Leave no one behind: ensuring access to COVID‐19 vaccines for refugee and displaced populations. Nat Med. 2021;27:747–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Harrison C. Honoring Latin America's Covid Vaccine Triumphs in 2021. Americas Society/Council of the Americas. 2021. [Google Scholar]
- 91. Guzman‐Holst A, DeAntonio R, Prado‐Cohrs D, Juliao P. Barriers to vaccination in Latin America: a systematic literature review. Vaccine. 2020;38:470–81. [DOI] [PubMed] [Google Scholar]
- 92. Margolis M. In Latin America, a Covid Vaccine Is No Panacea. 2020.
- 93. Cerda AA, García LY. Hesitation and refusal factors in individuals’ decision‐making processes regarding a Coronavirus Disease 2019 vaccination. Front Public Health. 2021;9:626852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Argote P, Barham E, Daly SZ, Gerez JE, Marshall J, Pocasangre O. The shot, the message, and the messenger: COVID‐19 vaccine acceptance in Latin America. NPJ Vaccines. 2021;6:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sallam M, Al‐Sanafi M, Sallam M. A global map of COVID‐19 vaccine acceptance rates per country: an updated concise narrative review. J Multidiscip Healthc. 2022;15:21–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mixed Migration Center . Quarterly Mixed Migration Update. MMC, 2021. [Google Scholar]
- 97. Chaves‐González D, Echevarría Estrada C. Venezuelan Migrants and Refugees in Latin America and the Caribbean: A Regional Profile. Organización Internacional para las Migraciones (OIM), 2020. [Google Scholar]
- 98. Perez‐Brumer A, Hill D, Andrade‐Romo Z, Solari K, Adams E, Logie C, et al. Vaccines for all? A rapid scoping review of COVID‐19 vaccine access for Venezuelan migrants in Latin America. J Migr Health. 2021;4:100072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Aranda Z, Arana‐Cedeño M, Meléndez‐Navarro DM, Meneses‐Navarro S, Sánchez‐Pérez HJ. A call for COVID‐19 immunization campaigns that address the specific circumstances of indigenous peoples of Latin America and the Caribbean. Lancet Reg Health Am. 2021;3:100074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lemos V. Covid: Viral photo highlights challenges of vaccinating Amazon. BBC. https://www.bbc.co.uk/news/world‐latin‐america‐59984857. Published January 13, 2022. Accessed January 26, 2022. [Google Scholar]
- 101. Hernández‐Vásquez A, Chavez‐Ecos F, Barrenechea‐Pulache A, Comandé D, Bendezu‐Quispe G. Seroprevalence and lethality by SARS‐CoV‐2 in indigenous populations of Latin America and the Caribbean: a systematic review. PeerJ. 2021;9:e12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bartlett J. Chile imposes lockdowns to fight new Covid wave despite vaccination success. The Guardian. http://www.theguardian.com/global‐development/2021/mar/28/chile‐coronavirus‐lockdowns‐vaccination‐success. Published March 28, 2021. Accessed April 13, 2021. [Google Scholar]
- 103. McKie R. Is vaccinating against Covid enough? What we can learn from Chile and Israel. The Guardian. http://www.theguardian.com/society/2021/apr/11/is‐vaccinating‐against‐covid‐enough‐what‐we‐can‐learn‐from‐other‐countries. Published April 11, 2021. Accessed April 13, 2021. [Google Scholar]
- 104. Ritchie H, Mathieu E, Rodés‐Guirao L, Appel C, Giattino C, Ortiz‐Ospina E, et al. Coronavirus Pandemic (COVID‐19). Our World in Data. 2020. [Google Scholar]
- 105. Nealon J, Cowling BJ. Omicron severity: milder but not mild. Lancet. 2022;399(10323):412–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re'em Y, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. UNESCO ‐ Institute for Statistics . Regional Dashboard – COVID‐19 Educational Response. UNESCO. [Google Scholar]
- 108. Hillis SD, Unwin HJT, Chen Y, Cluver L, Sherr L, Goldman PS, et al. Global minimum estimates of children affected by COVID‐19‐associated orphanhood and deaths of caregivers: a modelling study. Lancet. 2021;398:391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Perez‐Vincent SM, Carreras E. Domestic Violence Reporting during the COVID‐19 Pandemic: Evidence from Latin America. Inter‐American Development Bank, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. International Monetary Fund . Regional Economic Outlook. IMF, 2021. [Google Scholar]
- 111. Galarraga Gortázar N, Rivas Molina F, Fowkes J, Torrado S, Montes R. How South America became a global leader in Covid‐19 vaccination. El País. 2022. [Google Scholar]
- 112. Figar S, Pagotto V, Luna L, Salto J, Wagner Manslau M, Mistchenko AS, et al. Severe acute respiratory syndrome coronavirus 2019. Seroepidemiology study in Argentinian slum. Medicina (Mex). 2021;81:135–42. [PubMed] [Google Scholar]
- 113. Rodeles LM, Peverengo LM, Benítez R, Benzaquen N, Serravalle P, Long AK, et al. Seroprevalence of anti‐SARS‐CoV‐2 IgG in asymptomatic and pauci‐symptomatic people over a 5 month survey in Argentina. Rev Panam Salud Publica. 2021;45:e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Amorim Filho L, Szwarcwald CL, Mateos S de OG, Ponce de Leon ACM, Medronho R de A, Veloso VG, et al. Seroprevalence of anti‐SARS‐CoV‐2 among blood donors in Rio de Janeiro, Brazil. Rev Saude Publica. 2020;54:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Silveira MF, Barros AJD, Horta BL, Pellanda LC, Victora GD, Dellagostin OA, et al. Population‐based surveys of antibodies against SARS‐CoV‐2 in Southern Brazil. Nat Med. 2020;26:1196–9. [DOI] [PubMed] [Google Scholar]
- 116. Tess BH, Granato CFH, Alves MCGP, Pintão MCT, Nunes MC, Rizzatti EG, et al. Assessment of initial SARS‐CoV‐2 seroprevalence in the most affected districts in the municipality of São Paulo, Brazil. Braz J Infect Dis. 2021;25:101604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Hallal PC, Hartwig FP, Horta BL, Silveira MF, Struchiner CJ, Vidaletti LP, et al. SARS‐CoV‐2 antibody prevalence in Brazil: results from two successive nationwide serological household surveys. Lancet Glob Health. 2020;8:e1390–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. de Souza Araújo AA, Quintans‐Júnior LJ, Heimfarth L, Schimieguel DM, Corrêa CB, de Moura TR, et al. Seroprevalence of SARS‐CoV‐2 antibodies in the poorest region of Brazil: results from a population‐based study. Epidemiol Infect. 2021;149:e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Pasqualotto AC, Pereira P C de, Lana DFD, Schwarzbold AV, Ribeiro MS, Riche CVW, et al. COVID‐19 seroprevalence in military police force, Southern Brazil. PLoS One. 2021;16:e0249672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Silva AAM da, Lima‐Neto LG, Azevedo C de MP e S de, Costa LMM da, Bragança MLBM, Barros Filho AKD, et al. Population‐based seroprevalence of SARS‐CoV‐2 and the herd immunity threshold in Maranhão. Rev Saude Publica. 2020;54:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. do Couto AC, Kmetiuk LB, Delai RR, Brandão APD, Monteiro CO, da Silva LHA, et al. High SARS‐CoV‐2 seroprevalence in persons experiencing homelessness and shelter workers from a day‐shelter in São Paulo, Brazil. PLoS Negl Trop Dis. 2021;15:e0009754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Lalwani P, Salgado BB, Filho IVP, da Silva DSS, de Morais TB do N, Jordão MF, et al. SARS‐CoV‐2 seroprevalence and associated factors in Manaus, Brazil: baseline results from the DETECTCoV‐19 cohort study. Int J Infect Dis. 2021;110:141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Cristelli MP, Viana LA, Fortaleza CM, Granato C, Nakamura MR, Santos DWCL, et al. Lower seroprevalence for SARS‐CoV‐2‐specific antibodies among kidney transplant recipients compared to the general population in the city of Sao Paulo, Brazil. Transpl Infect Dis. 2021;23:e13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Miraglia JL, Nascimento Monteiro C, Giannecchini Romagnolo A, Xavier Gomes R, Pitangueiras Mangueira C, Aparecida Rosseto‐Welter E, et al. A seroprevalence survey of anti‐SARS‐CoV‐2 antibodies among individuals 18 years of age or older living in a vulnerable region of the city of São Paulo, Brazil. PLoS One. 2021;16:e0255412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Cavalcante Pinto Júnior V, Moura LFWG, Cavalcante RC, Lima JRC, Bezerra AS, de Sousa Dantas DR, et al. Prevalence of COVID‐19 in children, adolescents and adults in remote education situations in the city of Fortaleza, Brazil. Int J Infect Dis. 2021;108:20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Borges LP, Martins AF, de Melo MS, de Oliveira MGB, Neto JMR, Dósea MB, et al. Seroprevalence of SARS‐CoV‐2 IgM and IgG antibodies in an asymptomatic population in Sergipe, Brazil. Rev Panam Salud Publica. 2020;44:e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Malagón‐Rojas JN, Rubio V, Parra‐Barrera E. Seroprevalence and seroconversions for SARS‐CoV‐2 infections in workers at Bogota Airport, Colombia, 2020. J Travel Med. 2021;28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Mattar S, Alvis‐Guzman N, Garay E, Rivero R, García A, Botero Y, et al. Severe acute respiratory Syndrome Coronavirus 2 Seroprevalence among adults in a tropical city of the Caribbean Area, Colombia: are we much closer to herd immunity than developed countries? Open Forum Infect Dis. 2020;7:ofaa550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Colmenares‐Mejía CC, Serrano‐Díaz N, Quintero‐Lesmes DC, Meneses L, Salazar Acosta I, Idrovo ÁJ, et al. Seroprevalence of SARS‐CoV‐2 infection among occupational groups from the Bucaramanga Metropolitan Area, Colombia. Int J Environ Res Public Health. 2021;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Del Brutto OH, Costa AF, Mera RM, Recalde BY, Bustos JA, García HH. SARS‐CoV‐2 in Rural Latin America. A population‐based study in Coastal Ecuador. Clin Infect Dis. 2021;73:314–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Acurio‐Páez D, Vega B, Orellana D, Charry R, Gómez A, Obimpeh M, et al. Seroprevalence of SARS‐CoV‐2 infection and adherence to preventive measures in Cuenca, Ecuador, October 2020, a cross‐sectional study. Int J Environ Res Public Health. 2021;18:4657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Flamand C, Alves Sarmento C, Enfissi A, Bailly S, Beillard E, Gaillet M, et al. Seroprevalence of anti‐SARS‐CoV‐2 IgG at the first epidemic peak in French Guiana, July 2020. PLoS Negl Trop Dis. 2021;15:e0009945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Muñoz‐Medina JE, Grajales‐Muñiz C, Salas‐Lais AG, Fernandes‐Matano L, López‐Macías C, Monroy‐Muñoz IE, et al. SARS‐CoV‐2 IgG antibodies seroprevalence and sera neutralizing activity in MEXICO: a national cross‐sectional study during 2020. Microorganisms. 2021;9:850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Díaz‐Salazar C, Sánchez‐García A, Rodríguez‐Gutiérrez R, Camacho‐Ortiz A, Saldívar‐Rodríguez D, González‐González JG. Prevalence and associated characteristics of anti‐SARS‐CoV‐2 antibodies in Mexico 5 months after pandemic arrival. BMC Infect Dis. 2021;21:835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Cruz‐Arenas E, Cabrera‐Ruiz E, Laguna‐Barcenas S, Colin‐Castro CA, Chavez T, Franco‐Cendejas R, et al. Serological prevalence of SARS‐CoV‐2 infection and associated factors in healthcare workers in a “non‐COVID” hospital in Mexico City. PLoS One. 2021;16:e0255916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Rodríguez‐Vidales EP, Garza‐Carrillo D, Pérez‐Trujillo JJ, Robles‐Rodríguez OA, Salinas‐Martínez AM, Montes de Oca‐Luna R, et al. Prevalence of IgG antibodies induced by the SARS‐COV‐2 virus in asymptomatic adults in Nuevo Leon, Mexico. J Med Virol. 2021;93:5873–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Díaz‐Vélez C, Failoc‐Rojas VE, Valladares‐Garrido MJ, Colchado J, Carrera‐Acosta L, Becerra M, et al. SARS‐CoV‐2 seroprevalence study in Lambayeque, Peru. June‐July 2020. PeerJ. 2021;9:e11210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Huamaní C, Velásquez L, Montes S, Mayanga‐Herrera A, Bernabé‐Ortiz A. SARS‐CoV‐2 seroprevalence in a high‐altitude setting in Peru: adult population‐based cross‐sectional study. PeerJ. 2021;9:e12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Moreira‐Soto A, Pachamora Diaz JM, González‐Auza L, Merino Merino XJ, Schwalb A, Drosten C, et al. High SARS‐CoV‐2 Seroprevalence in Rural Peru, 2021: a Cross‐Sectional Population‐Based Study. mSphere. 2021;6:e0068521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. González S, Olszevicki S, Salazar M, Calabria A, Regairaz L, Marín L, et al. Effectiveness of the first component of Gam‐COVID‐Vac (Sputnik V) on reduction of SARS‐CoV‐2 confirmed infections, hospitalisations and mortality in patients aged 60–79: a retrospective cohort study in Argentina. EClinicalMedicine. 2021;40:101126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Hitchings MDT, Ranzani OT, Dorion M, D'Agostini TL, de Paula RC, de Paula OFP, et al. Effectiveness of ChAdOx1 vaccine in older adults during SARS‐CoV‐2 Gamma variant circulation in São Paulo. Nat Commun. 2021;12:6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Alencar CH, Cavalcanti LP de G, Almeida MM de, Barbosa PPL, Cavalcante KK de S, Melo DN de, et al. High effectiveness of SARS‐CoV‐2 vaccines in reducing COVID‐19‐related deaths in over 75‐year‐olds, Ceará State, Brazil. Trop Med Infect Dis. 2021;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Hitchings MDT, Ranzani OT, Torres MSS, de Oliveira SB, Almiron M, Said R, et al. Effectiveness of CoronaVac among healthcare workers in the setting of high SARS‐CoV‐2 Gamma variant transmission in Manaus, Brazil: a test‐negative case‐control study. Lancet Reg Health Am. 2021;1:100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Ranzani OT, Hitchings MDT, Dorion M, D'Agostini TL, de Paula RC, de Paula OFP, et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of covid‐19 in Brazil: test negative case‐control study. BMJ. 2021;374:n2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Jara A, Undurraga EA, González C, Paredes F, Fontecilla T, Jara G, et al. Effectiveness of an inactivated SARS‐CoV‐2 vaccine in Chile. N Engl J Med. 2021;385:875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Murillo‐Zamora E, Trujillo X, Huerta M, Riós‐Silva M, Mendoza‐Cano O. Effectiveness of BNT162b2 COVID‐19 vaccine in preventing severe symptomatic infection among healthcare workers. Medicina (Mex). 2021;57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Silvia Valencia J, Soto Becerra P, Escobar Agreda S, Fernández Navarro M, Moscoso Porras M, Solari L, et al. Efectividad de la vacuna BBIBP‐CorV para prevenir infección y muerte en personal de salud, Perú 2021. 2021.


