Abstract
Background and Aims
After 2 doses, the efficacy of anti‐SARS‐CoV‐2 vaccination seems to be lower in solid organ transplant recipients than in the immunocompetent population. The objective of this study was to determine the humoral response rate after vaccination, including with a booster dose, and to identify risk factors for non‐responsiveness in liver transplant recipients.
Methods
We included all patients seen in consultation in two French liver transplant centres between January 1, 2021, and March 15, 2021.
Results
598 liver transplant recipients were enrolled and 327 were included for analysis. Sixteen patients received one dose, 63 patients two doses and 248 patients three doses. Anti‐SARS‐Cov‐2 antibodies were detected in 242 out of 327 (74.0%) liver transplant patients after vaccination. Considering an optimal serologic response defined as an antibody titre >260 BAU/ml, 172 patients (52.6%) were responders. Mycophenolate mofetil (MMF) treatment was an independent risk factor for a failure to develop anti‐SARS‐CoV‐2 antibodies after vaccination (OR 0.458; 95%CI 0.258–0.813; p = .008). Conversely, male gender (OR 2.247, 95%CI 1.194–4.227; p = .012) and receiving an mRNA vaccine (vs a non‐mRNA vaccine) (OR 4.107, 95%CI 1.145–14.731; p = .030) were independent predictive factors for developing an optimal humoral response after vaccination. None of the patients who received the vaccine experienced any serious adverse events.
Conclusions
Even after a third booster dose, response rate to vaccination is decreased in liver transplant recipients. MMF appears to be a major determinant of seroconversion and optimal response to vaccination in these patients.
Keywords: liver transplant, mRNA vaccine, mycophenolate mofetil, SARS‐CoV‐2
Abbreviations
- BAU
Binding Antibody Units
- CI
Confidence interval
- CNIs
Calcineurin inhibitors
- COVID‐19
Coronavirus disease 2019
- IgG
Immunoglobulin G
- IQR
Interquartile range
- LT
liver transplant
- MMF
mycophenolate mofetil
- mRNA
messenger ribonucleic acid
- mTOR
Mammalian target of rapamycin
- RBD
Receptor binding domain
- SARS‐CoV‐2
Severe acute respiratory disease coronavirus 2
- SOT
Solid organ transplant
1. INTRODUCTION
Vaccination with mRNA vaccines (Pfizer‐BioNTech BNT162b2 and Moderna mRNA‐1273) protects 95% of immunocompetent patients from severe SARS‐CoV‐2 infection, 1 , 2 but solid organ transplant (SOT) recipients were excluded from these clinical trials. Indeed, the efficacy of anti‐SARS‐CoV‐2 vaccination seems to be lower in patients waiting for liver transplantation and liver transplant recipients than in the immunocompetent population, 3 , 4 with antibody responses following two doses ranging from 31% to 81.9% in liver transplant recipients. 4 , 5 , 6 , 7 , 8 Although response rates differ between studies, the main factors influencing negative serological responses tend to be consistent and include age, time from transplant and the immunosuppressive regimen used. 9 In particular, an Israeli study, 5 confirmed in other studies analysing the post‐vaccination humoral and cellular responses in liver transplant recipients, 10 , 11 reported significantly lower antibody titers in liver transplant recipients than healthy controls (95.41 AU/mL (n = 80) vs. 200.5 AU/ml (n = 25), p < .001) after two doses of the Pfizer‐BioNTech BNT162b2 vaccine. These poor humoral responses to COVID‐19 vaccination seen in liver transplant recipients may be due to treatment with high‐dose steroids or antimetabolites (e.g., mycophenolate mofetil; MMF), older age and lower estimated glomerular filtration rate.
The response to vaccination also depends on the organ transplanted. One study comparing kidney and liver transplant recipients confirmed a poorer vaccine response after two vaccine doses in kidney transplant recipients than in liver transplant recipients (58.5% vs 89.1%). 8 Another study showed that the administration of a third dose of the BNT162b2 vaccine to solid organ transplant recipients significantly improved vaccine immunogenicity. 12 , 13 These data prompted the recommendation of a third “booster” vaccination dose in patients without antibody responses after two vaccinations. 14 , 15
However, there are little data on vaccine efficacy after three vaccination doses in real‐life liver transplant recipients and the factors associated with serological responses at this stage. Thus, the objective of this study was to determine the humoral response rate after vaccination, including with a booster dose, and to identify risk factors for non‐responsiveness in liver transplant recipients.
2. PATIENTS AND METHODS
2.1. Study design
In this retrospective study, all adult (>18 years) liver transplant recipients seen in consultation were included in two French liver transplant centres: Montpellier St Eloi and Lyon Edouard Herriot. We collected clinical characteristics (age, sex and body mass index), comorbidities, the date of liver transplantation and immunosuppressive regimen. Date of vaccination, type of vaccine and serology post‐vaccination were also noted.
2.2. Antibody testing
Anti‐SARS‐Cov‐2 spike protein antibody detection was mostly performed using Elecsys Anti‐SARS‐CoV‐2 S (Roche) and SARS‐CoV‐2 IgG II Quant test (Abbott Laboratories) and converted to universal unit (BAU/ml). 16 Patients were stratified into three groups based on antibody levels (BAU/mL): <0.4 (non‐responders), 0.4–260 (partial responder), and >260 (responder). 17 For the univariate and multivariate analysis, patients were considered responders if antibodies >260 BAU/ml and non‐responders if <260 BAU/ml. 17
2.3. Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics version 28.0.0 (Inc., IL., USA). Continuous variables were presented as mean ± standard deviation. Categorical variables are presented as numbers and percentages. The relationship between categorical variables was assessed using the Chi‐square test of Pearson or Fisher exact test if the theoretical numbers were below 5. The connection between a qualitative and a quantitative variable was evaluated using the Student’s t test or the ANOVA test. The variables that had a p value <.20 in univariate analysis and those that had clinical relevance have been introduced in the multivariate analysis. The binary logistic regression model was used to identify independent predictive factors influencing the serologic response after the COVID vaccine. For all tests, statistical significance is set at p < .05.
All procedures were conducted in accordance with the appropriate ethics and/or institutional review committee(s). The study was approved by the institutional review board (IRB, 202100907). Informed consent was obtained verbally and the IRB reviewed and approved the verbal consent process that was recorded in the electronic medical records.
3. RESULTS
3.1. Liver transplant recipient characteristics
Between January 1, 2021, and March 15, 2021, 598 liver transplant recipients were enrolled in the study, 495 from centre 1 and 103 from centre 2. Of these, 103 received no anti‐SARS‐CoV‐2, 13 had missing data (2 of which were diagnosed with Covid‐19 disease during vaccination), and 155 were excluded from the analysis because they had serology within 28 days of vaccination (Figure 1). Therefore, data on anti‐SARS‐CoV‐2 vaccination and serology four weeks after the last dose were available for 327 patients. Sixteen patients received one dose, 63 patients two doses and 248 patients three doses. Most patients (308, 94.2%) received an anti‐SARS‐CoV‐2 mRNA vaccine (Pfizer‐BioNTech BNT162b2 or Moderna mRNA‐1273) and 19 received AZD1222 (Vaxzevria, AstraZeneca).
FIGURE 1.
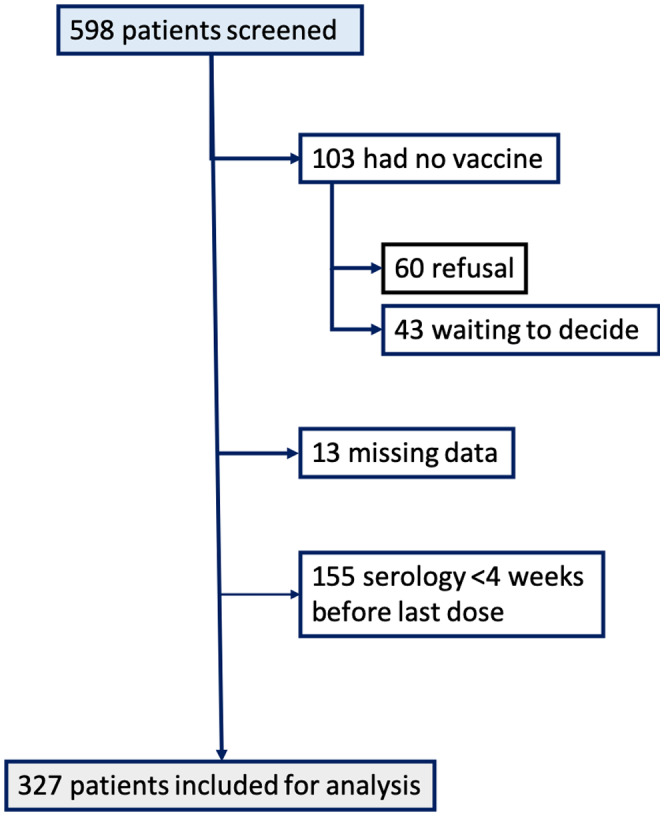
Flow chart of patient inclusion
The patient characteristics and their immunosuppressive regimens are shown in Table 1. A majority of patients were men (214, 65.4%), with a mean age of 60 ± 13 years. The mean time from transplantation to the first vaccine dose was 7.60 ± 7.78 years. The most common indication for liver transplantation was alcohol‐related liver disease (119, 36.4%), with the other indications listed in Table 1. Calcineurin inhibitors (CNIs) were used in 258 (79.1%) patients, mammalian target of rapamycin (mTOR) inhibitors in 62 patients (19.0%), and an antimetabolite (i.e., MMF) in 194 patients (59.5%). About half of patients (173, 52.9%) were treated with a combination of two immunosuppressive therapies, while 124 (37.9%) received monotherapy and 25 (7.6%) received triple therapy. Twenty patients (6.1%) had a history of COVID‐19.
TABLE 1.
Characteristics of the study population
| Gender, n (%) | |
| Women | 113 (34.6%) |
| Men | 214 (65.4%) |
| Age (years), mean (SD) [max‐min] | 60 (13) [83–18] |
| Aetiology | |
| Alcohol | 119 (36.4%) |
| NASH | 21 (6.4%) |
| HCC | 11 (3.4%) |
| Auto‐immune (PBC/AIH/PSC) | 46 (14.1%) |
| Others | 130 (39.8%) |
| Type of organ transplant, n (%) | |
| Liver | 316 (96.6%) |
| Liver + kidney | 9 (2.8%) |
| Liver + heart | 2 (0.6%) |
| Number of liver transplants, n (%) | |
| 1 | 312 (95.4%) |
| 2 | 14 (4.3%) |
| 3 | 1 (0.3%) |
| Diabetes mellitus, n (%) | |
| No DM | 211 (64.7%) |
| DM | 115 (35.3%) |
| Body mass index (kg/m2), mean (SD) [max‐min] | 26.32 (5.57) [41.00–15.79] |
| Time since transplantation (years), mean (SD) [max‐min] | 7.60 (7.78) [33.00–0.00] |
| Calcineurin inhibitor use, n (%) | |
| No calcineurin inhibitors | 68 (20.9%) |
| Calcineurin inhibitors | 258 (79.1%) |
| Mycophenolate mofetil use, n (%) | |
| No mycophenolate mofetil | 132 (40.5%) |
| Mycophenolate mofetil | 194 (59.5%) |
| Corticosteroid use, n (%) | |
| No corticosteroid | 287 (88.0%) |
| Corticosteroid | 39 (12.0%) |
| mTOR inhibitors, n (%) | |
| No mTOR | 264 (81.0%) |
| mTOR | 62 (19.0%) |
| Number of immunosuppressive therapies, n (%) | |
| 1 | 124 (37.9%) |
| 2 | 173 (52.9%) |
| 3 | 25 (7.6%) |
| 4 | 2 (0.6%) |
3.2. SARS‐CoV‐2 vaccination efficacy
Anti‐SARS‐Cov‐2 antibodies were detected in 242 out of 327 (74.0%) liver transplant patients after vaccination. With an optimal serologic response defined as an antibody titre >260 BAU/ml, 172 patients (52.6%) were responders and 70 (21%) had a partial serologic response (0.4 < antibody <260). In univariate analysis, history of COVID, MMF treatment and vaccine type predicted vaccine response (Table 2). In a multivariate model including gender, age, diabetes mellitus, body mass index, history of COVID, time since liver transplant, type of liver transplant, calcineurin inhibitor use, MMF use, corticosteroid use, mTOR inhibitor use, number of immunosuppressive therapies, type of vaccine and number of vaccine doses, MMF treatment was an independent risk factor for a failure to develop anti‐SARS‐CoV‐2 antibodies after vaccination (OR 0.458; 95%CI 0.258–0.813; p = .008). Conversely, male gender (OR 2.247, 95%CI 1.194–4.227; p = .012) and receiving an mRNA vaccine (vs a non‐mRNA vaccine) (OR 4.107, 95%CI 1.145–14.731; p = 0.030) were independent predictive factors for developing an optimal humoral response after vaccination (Table 3).
TABLE 2.
Clinical and biological characteristics of solid organ transplant recipients according to humoral response after SARS‐CoV‐2 vaccination
| No serologic response (n = 155) | Optimal serologic response (n = 172) | p‐value | |
|---|---|---|---|
| Gender, n (%) | .156 | ||
| Women | 60 (38.7) | 53 (30.8) | |
| Men | 95 (61.3) | 119 (69.2) | |
| Age (years), mean (SD) | 60 (13) | 59 (14) | .254 |
| Diabetes mellitus, n (%) | .697 | ||
| No DM | 103 (66.5) | 109 (63.4) | |
| DM | 52 (33.5) | 63 (36.6) | |
| Body mass index (kg/m2), mean (SD) | 26.25 (4.75) (26.38 (6.17) | .424 | |
| Previous history of COVID‐19 disease, n (%) | .036 | ||
| No COVID‐19 | 150 (96.8) | 157 (91.3) | |
| COVID‐19 | 5 (3.2) | 15 (8.7) | |
| Aetiology, n (%) | .125 | ||
| Alcohol | 53 (34.2) | 66 (38.4) | |
| NASH | 7 (4.5) | 14 (8.1) | |
| HCC | 3 (1.9) | 8 (4.7) | |
| Auto‐immune (PBC/AIH/PSC) | 21 (13.5) | 25 (14.5) | |
| Other | 71 (45.8) | 59 (34.3) | |
| Time since transplant (years), mean (SD) | 8.07 (8.02) | 7.17 (7.56) | .261 |
| Type of organ transplant, n (%) | .39 | ||
| Liver | 151 (97.4) | 165 (52.2) | |
| Liver + kidney | 4 (2.6) | 5 (55.6) | |
| Liver + heart | 0 (0.0) | 2 (100.0) | |
| Number of liver transplants, n (%) | .078 | ||
| 1 | 151 (97.4) | 161 (94.6) | |
| 2 | 3 (1.9) | 11 (6.4) | |
| 3 | 1 (0.7) | 0 (0.0) | |
| Calcineurin inhibitor use (/326), n (%) | .488 | ||
| No calcineurin inhibitors | 30 (19.4) | 38 (22.1) | |
| Calcineurin inhibitors | 125 (80.6) | 133 (77.3) | |
| Mycophenolate mofetil use (/326), n (%) | .006 | ||
| No mycophenolate mofetil | 51 (32.9) | 81 (47.1) | |
| Mycophenolate mofetil | 104 (67.1) | 90 (52.3) | |
| Corticosteroid use (/326), n (%) | .648 | ||
| No corticosteroid | 135 (87.1) | 152 (88.4) | |
| Corticosteroid | 20 (12.9) | 19 (11.0) | |
| mTOR inhibitor use (/326), n (%) | .3 | ||
| No mTOR | 129 (83.2) | 135 (78.5) | |
| mTOR | 26 (16.8) | 36 (20.9) | |
| Number of immunosuppressive therapies (/324), n (%) | .351 | ||
| 1 | 62 (40.0) | 62 (36.0) | |
| 2 | 83 (53.5) | 90 (52.3) | |
| 3 | 9 (5.8) | 16 (9.3) | |
| 4 | 1 (0.7) | 1 (0.6) | |
| Time between the last injection and COVID serology (days), mean (SD) | 46 (28) | 46 (26) | .348 |
| Number of immunosuppressive therapies, mean (SD) | 1.67 (0.61) | 1.71 (0.68) | .36 |
| Type of COVID vaccine (RNA vs protein), n (%) | .019 | ||
| Protein alone | 14 (9.0) | 5 (2.9) | |
| RNA or combined | 141 (91.0) | 167 (97.1) | |
| Number of vaccine doses, mean (SD) | 2.74 (0.55) | 2.69 (0.56) | .238 |
| Number of vaccine doses, n (%) | .522 | ||
| 1 | 8 (5.2) | 8 (4.7) | |
| 2 | 25 (16.1) | 38 (22.1) | |
| 3 | 122 (78.7) | 126 (73.2) | |
| 4 | 0 (0.0) | 0 (0.0) |
TABLE 3.
Multivariate logistic regression model evaluating clinical predictors for adequate qualitative spike IgG serology in LT recipient
| Clinical characteristics | OR | IC 95 | p value | ||
|---|---|---|---|---|---|
| Association with serologic response | Male gender | 2.247 | 1.194 | 4.227 | .012 |
| History of COVID disease | 2.881 | 0.873 | 9.513 | .083 | |
| MMF treatment | 0.458 | 0.258 | 0.813 | .008 | |
| RNA vaccine (vs non‐RNA vaccine) | 4.107 | 1.145 | 14. 731 | .030 | |
3.3. SARS‐CoV‐2 vaccination safety and acceptance
None of the patients who received the vaccine experienced any serious adverse events. Of the 598 patients who were offered a COVID‐19 vaccination between January 1, 2021, and March 15, 2021, 103 did not receive the vaccine, 60 due to refusal and 43 because they were “waiting to decide”.
4. DISCUSSION
Several studies have reported a higher risk of severe COVID‐19 disease and related mortality in SOT recipients and a lower vaccine response in these patients. 12 , 16 To our knowledge, there are no data on humoral responses to three doses of the SARS‐CoV‐2 vaccine in liver transplant recipients. In this retrospective study, we aimed to determine factors predictive of a humoral response to anti‐SARS‐CoV‐2 vaccination in a large cohort of liver transplant recipients. 327 LT recipients were vaccinated against SARS‐CoV‐2, mostly with three doses. We found: (1) a seroconversion rate of 74%; (2) a vaccine response rate of 52.6% at a response threshold of >260 BAU/ml; (3) a non‐RNA vaccination strategy was strongly associated with a poorer response; and (4) MMF was an independent predictor of a failure to mount a humoral response after vaccination.
While a few studies have assessed humoral responses to two doses of mRNA vaccine in SOT patients, 4 , 5 , 6 , 7 there are little data on serological responses after three doses. Kamar et al. reported an increase in the number of seropositive patients after the third dose of vaccine in SOT recipients (26/59 patients, 44%). 12 In their report of a cohort of SOT recipients receiving a third mRNA‐based vaccination, Del Bello et al. found seropositivity in 41.4% of patients after the second dose and an increase to 67.9% after the third dose. 13 In their cohort, liver transplant recipients were poorly represented compared with other SOT recipients, and indeed several studies have shown that the vaccine response differs according to the solid organ transplanted. 8 Several studies have shown a relatively high rate of vaccine immunogenicity in liver transplant patients compared with other organ transplant patients, suggesting that the liver transplant population might respond better to vaccination. 5 , 17
Despite seroconversion in 74% of our cohort, only 52.6% of patients had an optimal antibody level (> 260 BAU/ml) and a quarter of patients had no response, worse than expected for this population. Existing data on vaccine responses have not taken thresholds proposed to differentiate between partial and optimal responders into account. 18 For non‐responders, protection strategies are limited to a fourth dose or an antibody cocktail (such as casirivimab‐imdevimab) for COVID‐19 prophylaxis, 19 although the evolution of SARS‐CoV‐2 variants has led to a preference for tixagevimab/cilgavimab as prophylaxis in non‐responders or partial responders. 20 It is therefore important to be able to predict non‐response to vaccination so that extra modifiable risk factors can be addressed or extra protection administered. In our study, MMF was a strong modifiable risk factor for a lack of vaccine response (OR 0.458, 95%CI 0.258–0.813; p = .008), consistent with previous studies of heterogeneous SOT groups. 21 , 22 Recently, Timmermann et al. reported vaccine responses in 118 liver transplant patients after two doses, of whom 92 developed anti‐spike protein IgG antibodies (78%), 10 with alcohol‐induced cirrhosis and MMF risk factors for a failure to develop antibodies after vaccination. In their study, patients were not classified according to antibody levels, even though it is now known that poor responders to vaccination are at risk of severe COVID‐19 disease. 10 To our knowledge, there is no prospective study evaluating the MMF discontinuation before vaccination on the humoral response in LT recipients. Previously, the link between MMF and more severe COVID disease has been demonstrated. 23 The mechanism of action of MMF inhibiting antibody formation may explain the reduced humoral response and the occurrence of more severe COVID disease. We also found that a non‐mRNA vaccine (vs mRNA vaccine) (OR 4.107, 96%CI 1.145–14.731, p = .030) was a second modifiable predictor of vaccine non‐responsiveness. Confirmation of our results in other cohorts could inform recommendations on the best type of vaccine to use in solid organ transplant recipients.
Eighty‐three per cent of our population were vaccinated, a lower proportion than reported by others 24 , 25 and only 10% higher than the French national average of 74.6% on October 31, 2021. This can be explained by a particularly high level of mistrust of vaccines in France, which was present before and exacerbated by the pandemic. The reasons for refusing the vaccine are often multiple and may be political or related to irrational beliefs. We did not specifically investigate adverse events related to vaccination, which were often minor, but no serious post‐vaccination adverse events were reported.
Study limitations are usual for retrospective studies with a lack of data. Some data could not be collected such as smoking status and renal function. Futhermore, the use of two different methodologies to test humoral response could be a source of classification bias. Several teams, including recently Kamar et al, 26 , 27 have shown a decrease in antibodies in SOT recipients after vaccination between 1 and 3 months. In our study, data on the longitudinal follow‐up of the humoral response were not available. This is a limitation for the interpretation of the long‐term vaccine response. Finally, in order to improve the analysis of the factors associated with the vaccine response, it would have been interesting to have the serology after each vaccine dose.
In conclusion, after three doses of vaccine, about half of the liver transplant recipients have an optimal response. MMF appears to be a major determinant of seroconversion and optimal response to vaccination in liver transplant recipients. The nature of immunosuppression must be considered to improve vaccine responses in liver transplant recipients and anti‐SARS‐CoV‐2 mRNA vaccines are preferred in this population.
CONFLICT OF INTEREST
No conflicts of interest related to this article required declaration by the authors. Funding support came solely from institutional and/or departmental sources (institute: Montpellier University Hospital, 34 000, France).
Meunier L, Sanavio M, Dumortier J, et al. Mycophenolate mofetil decreases humoral responses to three doses of SARS‐CoV‐2 vaccine in liver transplant recipients. Liver Int. 2022;42:1872–1878. doi: 10.1111/liv.15258
The study was approved by the institutional review board (IRB, 202100907)
Handling Editor: Alejandro Forner
REFERENCES
- 1. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383(27):2603‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2021;384(5):403‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benotmane I, Gautier G, Perrin P, et al. Antibody response after a third dose of the mRNA‐1273 SARS‐CoV‐2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA. 2021;23:1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2‐dose SARS‐CoV‐2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204‐2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rabinowich L, Grupper A, Baruch R, et al. Low immunogenicity to SARS‐CoV‐2 vaccination among liver transplant recipients. J Hepatol. 2021;75(2):435‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guarino M, Cossiga V, Esposito I, Furno A, Morisco F. Effectiveness of SARS‐CoV‐2 vaccination in liver transplanted patients: the debate is open! J Hepatol 2022;76(1):237–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ruether DF, Schaub GM, Duengelhoef PM, Haag F, Brehm TT, Fathi A, et al. SARS‐CoV2‐specific humoral and T‐cell immune response after second vaccination in liver cirrhosis and transplant patients. Clin Gastroenterol Hepatol 2022;20(1):162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nazaruk P, Monticolo M, Jędrzejczak AM, et al. Unexpectedly high efficacy of SARS‐CoV‐2 BNT162b2 vaccine in liver versus kidney transplant recipients‐is it related to immunosuppression only? Vaccines. 2021;9(12):1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rabinowich L, Shibolet O, Katchman H. Reply to: "Effectiveness of SARS‐CoV‐2 vaccination in liver transplanted patients: The debate is open!". J Hepatol 2022;76(1):239–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Timmermann L, Globke B, Lurje G, et al. Humoral immune response following SARS‐CoV‐2 vaccination in liver transplant recipients. Vaccines. 2021;9(12):1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. D’Offizi G, Agrati C, Visco‐Comandini U, et al. Coordinated cellular and humoral immune responses after two‐dose SARS‐CoV2 mRNA vaccination in liver transplant recipients. Liver Int. 2021;31:180‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA Covid‐19 vaccine in solid‐organ transplant recipients. N Engl J Med. 2021;385(7):661‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Del Bello A, Abravanel F, Marion O, et al. Efficiency of a boost with a third dose of anti‐SARS‐CoV‐2 messenger RNA‐based vaccines in solid organ transplant recipients. Am J Transplant. 2021;31:322‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DGS‐Urgent . Vaccins contre la Covid‐19: modalites d’administration des rappels. 2021. (https://www.mesvaccins .net/ textes/dgs_urgent_n43_vaccination_modalites_d_administration_des_rappels.pdf).
- 15. Barda N, Dagan N, Cohen C, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID‐19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet Lond Engl. 2021;398(10316):2093‐2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dumortier J, Duvoux C, Roux O, et al. Covid‐19 in liver transplant recipients: the French SOT COVID registry. Clin Res Hepatol Gastroenterol. 2021;45(4):101639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marion O, Del Bello A, Abravanel F, et al. Predictive factors for humoral response after 2‐dose SARS‐CoV‐2 vaccine in solid organ transplant patients. Transplant Direct. 2022;8(1):e1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feng S, Phillips DJ, White T, et al. Correlates of protection against symptomatic and asymptomatic SARS‐CoV‐2 infection. Nat Med. 2021;27(11):2032‐2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN‐COV2, a neutralizing antibody cocktail, in outpatients with Covid‐19. N Engl J Med. 2021;384(3):238‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tixagevimab and Cilgavimab (Evusheld) for pre‐exposure prophylaxis of COVID‐19. JAMA. 2022;327(4):384‐385. [DOI] [PubMed] [Google Scholar]
- 21. Marinaki S, Adamopoulos S, Degiannis D, et al. Immunogenicity of SARS‐CoV‐2 BNT162b2 vaccine in solid organ transplant recipients. Am J Transplant. 2021;21(8):2913‐2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marion O, Del Bello A, Abravanel F, et al. Safety and immunogenicity of anti‐SARS‐CoV‐2 messenger RNA vaccines in recipients of solid organ transplants. Ann Intern Med. 2021;174(9):1336‐1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Colmenero J, Rodríguez‐Perálvarez M, Salcedo M, et al. Epidemiological pattern, incidence, and outcomes of COVID‐19 in liver transplant patients. J Hepatol. 2021;74(1):148‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Giannini EG, Marenco S. High acceptance rate of COVID‐19 vaccination in liver transplant recipients. J Hepatol. 2021;75(2):483‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Costantino A, Invernizzi F, Centorrino E, Vecchi M, Lampertico P, Donato MF. COVID‐19 vaccine acceptance among liver transplant recipients. Vaccines. 2021;9(11):1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kamar N, Abravanel F, Marion O, et al. Anti‐SARS‐CoV‐2 spike protein and neutralizing antibodies at 1 and 3 months after three doses of SARS‐CoV‐2 vaccine in a large cohort of solid organ transplant patients. Am J Transplant. 2022;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Caballero‐Marcos A, Citores MJ, Alonso‐Fernández R, et al. Decreased long‐term severe acute respiratory syndrome coronavirus 2‐specific humoral immunity in liver transplantation recipients 12 months after coronavirus disease 2019. Liver Transplant. 2021;17. [DOI] [PubMed] [Google Scholar]


