Abstract
Background
Candida auris is an emerging multidrug‐resistant pathogen in intensive care settings (ICU). During the coronavirus disease 19 (COVID‐19) pandemic, ICU admissions were overwhelmed, possibly contributing to the C. auris outbreak in COVID‐19 patients.
Objectives
The present systematic review addresses the prevalence, underlying diseases, iatrogenic risk factors, treatment and outcome of C. auris infections in COVID‐19 patients.
Methods
MEDLINE, Scopus, Embase, Web of Science and LitCovid databases were systematically searched with appropriate keywords from 1 January 2020 to 31 December 2021.
Results
A total of 97 cases of C. auris were identified in COVID‐19 patients. The pooled prevalence of C. auris infections (encompassing candidemia and non‐candidemia cases) in COVID‐19 patients was 14%. The major underlying diseases were diabetes mellitus (42.7%), hypertension (32.9%) and obesity (14.6%), followed by the iatrogenic risk factors such as a central venous catheter (76.8%%), intensive care unit (ICU) stay (75.6%) and broad‐spectrum antibiotic usage (74.3%). There were no significant differences in underlying disease and iatrogenic risk factors among C. auris non‐candidemia/colonisation and C. auris candidemia cases. The mortality rate of the total cohort is 44.4%, whereas, in C. auris candidemia patients, the mortality was 64.7%.
Conclusion
This study shows that the prevalence of C. auris infections remains unchanged in the COVID‐19 pandemic. Hospital‐acquired risk factors may contribute to the clinical illness. Proper infection control practices and hospital surveillance may stop future hospital outbreaks during the pandemic.
Keywords: Candida auris, candidemia, COVID‐19, mortality, prevalence, systematic review
1. INTRODUCTION
During the coronavirus disease 19 (COVID‐19) pandemic, the healthcare facility was overwhelmed with patients admitted to intensive care units (ICUs), and those patients were highly susceptible to bacterial and fungal co‐infections. 1 , 2 Candida auris is an emerging pathogen in ICU settings with high mortality and infection due to this pathogen has been reported across 44 countries. 3 , 4 Candida auris is a unique species, as this agent is multidrug‐resistant, and they can survive on inanimate objects in the hospital environment for more extended periods, leading to potential transmission among patients (hospital outbreaks), and difficulty in accurate laboratory identification makes them the pathogen of public interest globally. 5 , 6 , 7 , 8 Patients with underlying diseases such as diabetes mellitus, kidney disease, lung disease, trauma, ear diseases and hypertension, followed by iatrogenic risk factors such as prolonged ICU stay, central venous catheter, mechanical ventilation and prior antibiotic usage, were significantly associated with C. auris infections prior to the COVID‐19 pandemic. 3 , 4 , 9 , 10
Like C. auris infections, diabetes mellitus, hypertension, malignancies, chronic kidney diseases, chronic liver disease and cardiovascular disease are associated with high mortality in severe COVID‐19 patients. 11 , 12 Underlying disease and the invasive medical procedures during the hospital stay in COVID‐19 patients make them highly susceptible to C. auris infections/colonisation. The outbreak of C. auris infections has been reported during the COVID‐19 pandemic across different countries. 13 , 14 , 15 , 16 Due to the overlapping features (underlying disease/risk factors) between the two disease groups, the disease epidemiology of C. auris infections in COVID‐19 patients will be interesting to study. In the present study, we systematically reviewed the C. auris infections/colonisation in COVID‐19 patients to determine the prevalence, underlying disease/risk factors, treatment and outcome.
2. METHODOLOGY
2.1. Literature search and eligibility criteria
The proposal for the present systematic review was registered with PROSPERO (registration number: CRD42021252484). The systematic review was conducted as per PRISMA guidelines. The following databases (MEDLINE, Scopus, Embase, Web of Science, LitCovid, back‐reference of the manuscripts) were searched for articles published in the English language from 1 January 2020 to 31 December 2021. The study design is described in Figure 1. Studies encompassing details of C. auris infection in COVID‐19 patients, such as case reports, case series (≥2 cases), retrospective cohort studies and observational studies, were included in the review. Studies that failed to fulfil the inclusion criteria were excluded (infections other than C. auris, review articles, articles other than English and studies without details of the study population were excluded from the review).
FIGURE 1.
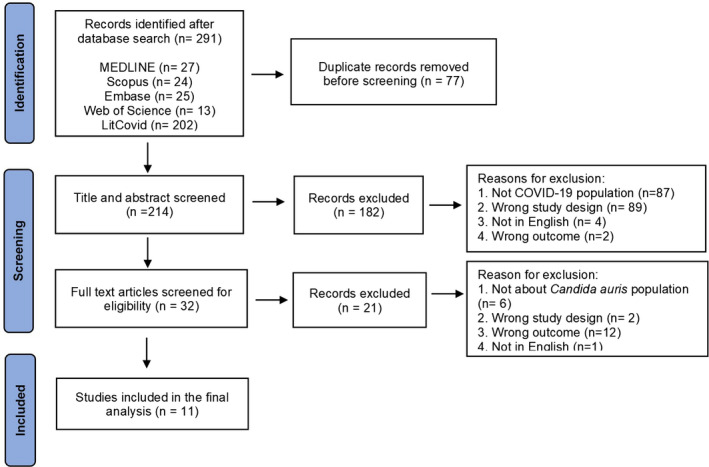
PRISMA flowchart describing the study selection process
2.2. Study selection and data extraction
After the literature search, the citations were uploaded to Rayyan QCRI software for screening. 17 Following duplicates removal, the title and abstract of the articles were screened for inclusion by two independent reviewers (KSV and HPP), and disparities were sorted by discussion and consensus (sought the opinion of third reviewer KCP); the qualified articles were screened for full text by two independent reviewers (KSV and HPP). Only the studies that qualified after full‐text screening were proceeded to the data extraction process by two independent reviewers (KSV and HPP) and verified by a third reviewer (KCP). The following data were extracted in Microsoft excel: study design, country of the study, number of C. auris cases reported, patients details such as age, sex, underlying diseases, iatrogenic risk factors, antifungal treatment and outcome, that is, mortality, details of clinical specimens, antifungal susceptibility results and drug‐resistance of the C. auris isolates. Further, for estimation of pooled prevalence of C. auris infections in COVID‐19 patients, only the studies that provided information on total number of C. auris cases and total number of COVID‐19 cases were included in the analysis (studies without denominators such as case reports and case series were excluded from the prevalence estimation analysis).
2.3. Risk of bias assessment
The risk of bias assessment was done by two independent reviewers (KSV and HPP) using a modification of the Joanna Briggs Institute (JBI) tool for the case series. 18 , 19 The risk of bias was assessed as low, high and unclear under the following domains: clear inclusion criteria, a valid identification method, clear reporting of the demographic information, clinical parameters, outcomes, the presenting site(s)/clinic(s) demographic information.
2.4. Statistical methods
Comparison of underlying diseases, iatrogenic risk factors, antifungal therapy and the outcome of the disease between the C. auris non‐candidemia/colonisation and C. auris candidemia cases was performed using Fisher's exact test using MedCalc Statistical Software (MedCalc Statistical Software version 14.8.1 (MedCalc Software by, Ostend, Belgium; http://www.medcalc.org; 2014)). ‘p’ values <.05 was considered significant. Meta‐analysis was done using OpenMeta analyst software (version 10.10). 20 , 21 Pooled prevalence was calculated using the binary random effect model (Restricted Maximum likelihood method). Heterogeneity among the studies was evaluated using I 2 statistics.
3. RESULTS
The initial database search identified 291 articles; of those, 11 articles were included in the final analysis (Figure 1), and the data were extracted for qualitative and quantitative analysis. Quality assessment of the articles was done using the JBI tool; of the 11 articles assessed, 8 fulfilled the criteria as mentioned above, 13 , 14 , 16 , 22 , 23 , 24 , 25 , 26 in 3 of the articles, the method of identification was unclear, and the rest of the criteria were fulfilled. 15 , 27 , 28 A total of 97 cases of C. auris were identified in COVID‐19 patients across different countries (Figure 2). Majority of the cases were reported from the United States of America (n = 48, 49.5%), followed by Mexico (n = 12, 12.4%) and India (n = 10, 10.3%; Figure 2). The male to female ratio was 2.6:1. The mean age was 65.41 years (with a range of 1–101 years), and 99% (n = 96) of the patients with C. auris infections were adults (Table 1). For estimation of pooled prevalence of C. auris infections in the COVID‐19 patients, the data from the 5 studies were analysed (Figure 3). 15 , 16 , 23 , 25 , 26 The prevalance of C. auris infections (including candidemia and non‐candidemia cases) among the COVID‐19 patients was 14%, with high heterogeneity among the included publications (I 2 = 99.88; Figure 3).
FIGURE 2.
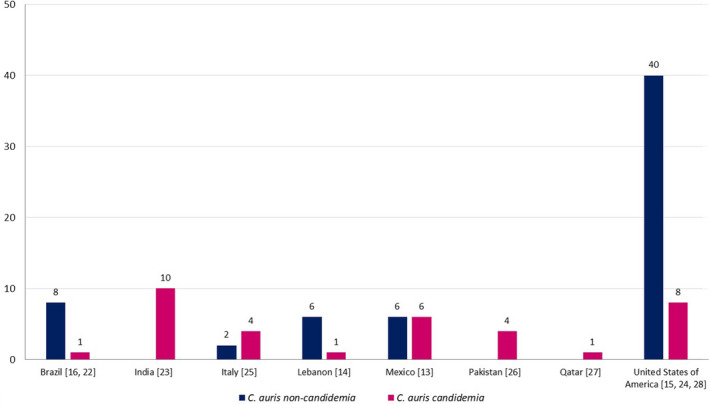
Candida auris cases in COVID‐19 patients across countries. References are given in square brackets
TABLE 1.
Risk factors, treatment and outcome of Candida auris cases in COVID‐19 patients
| Study Parameters | Total cohort (TC) of Patients [n (%)] a | Candida auris non‐candidemia (CANC) cases in COVID‐19 patients | Candida auris candidemia (CAC) cases in COVID‐19 patients | p value |
|---|---|---|---|---|
| Candida auris in COVID‐19 patients | 97 (100) | 62 | 35 | ‐ |
| Age in years (range) | 1–101 | 36–89 b | 1–86 b | ‐ |
| Mean age (in years) (n = 58) c | 65.41 c | 65.6 c | 65.3 c | ‐ |
| Male:Female ratio (n) | 2.6:1 (70:27) | 3.5:1 (21:6) b | 4:1 (28:7) b | ‐ |
| Paediatric | 1 | 0 b | 1 b | ‐ |
| Adult | 96 | 27 b | 34 b | ‐ |
| Mean days from admission to first isolation of Candida auris (n = 58) c | 25.1 c | 27.7 c | 22.8 c | ‐ |
| The values are expressed in numbers and percentage [n (%)] | ||||
| Clinical Specimens and Candida auris Isolation b | TC (n = 62) b | CANC Cases (n = 27) b | CAC cases (n = 35) b | p value |
|---|---|---|---|---|
| Blood | 35 (56.5) | 0 | 35 (100) | ‐ |
| Urine | 12 (19.4) | 10 (37.0) | 2 (5.7) | ‐ |
| Deep tracheal aspirate and broncho alveolar lavage | 10 (16.1) | 9 (33.3) | 1 (2.9) | ‐ |
| Axillae | 6 (9.7) | 6 (22.2) | 0 | ‐ |
| Groin | 5 (8.1) | 5 (18.5) | 0 | ‐ |
| Skin swab and wound specimens | 5 (4.8) | 3 (11.1) | 2 (5.7) | ‐ |
| Nostrils | 3 (4.8) | 3 (11.1) | 0 | ‐ |
| Central venous catheter tip | 3 (4.8) | 2 (7.4) | 1 (2.9) | ‐ |
| Ear swab | 2 (3.2) | 2 (7.4) | 0 | ‐ |
| Underlying diseases | TC (n = 82) a | CANC cases (n = 27) b | CAC cases (n = 35) b | p value |
|---|---|---|---|---|
| Diabetes mellitus | 35 (42.7) | 11 (40.7) | 12 (34.3) | .79 |
| Hypertension | 27 (32.9) | 10 (37) | 17 (48.6) | .44 |
| Obesity | 12 (14.6) | 8 (29.6) | 4 (11.4) | .106 |
| Immunocompetent | 10 (12.2) | 2 (7.4) | 4 (11.4) | .689 |
| COVID‐19 associated acute respiratory distress syndrome (ARDS) | 9 (11) | 6 (22.2) | 3 (8.6) | .16 |
| Malignancy | 8 (13.4) | 3 (11.1) | 2 (5.7) | .645 |
| Renal diseases d | 11 (13.4) | 2 (7.4) | 6 (17.1) | .447 |
| Heart disease e | 8 (9.6) | 3 (11.1) | 4 (11.4) | >.99 |
| Liver and biliary disease f | 4 (4.9) | 1 (3.7) | 3 (8.6) | .626 |
| Thromboembolic disease g | 5 (6.1) | 3 (11.1) | 2 (5.7) | .645 |
| Miscellaneous respiratory diseases h | 9 (11) | 5 (18.5) | 4 (11.4) | .485 |
| Others i | 15 (18.3) | 7 (25.9) | 4 (11.4) | .185 |
| Iatrogenic risk factors | TC (n = 82) a | CANC cases (n = 27) b | CAC cases (n = 35) b | p value |
|---|---|---|---|---|
| Central venous catheter | 63 (76.8) | 19 (70.3) | 28 (80) | .551 |
| Intensive care unit (ICU) stay | 62 (75.6) | 27 (100) | 33 (94.3) | .5 |
| Broad spectrum antibiotic usage | 61 (74.3) | 26 (96.3) | 35 (100) | .436 |
| Mechanical ventilation | 57 (69.5) | 22 (81.5) | 24 (86.6) | .381 |
| Steroid therapy | 51 (62.2) | 24 (88.9) | 27 (77.1) | .321 |
| Urinary catheter | 47 (57.3) | 17 (63) | 19 (54.3) | .606 |
| Co‐infections along with C. auris | 43 (52.4) | 13 (48.1) | 25 (71.4) | .12 |
| Previous antifungal exposure | 19 (23.2) | 12 (44.4) | 7 (20) | .053 |
| Haemodialysis | 7 (8.5) | 3 (11.1) | 4 (11.4) | >.99 |
| Antifungal therapy b | TC (n = 62) b | CANC cases (n = 27) b | CAC cases (n = 35) b | p value |
|---|---|---|---|---|
| Patients received antifungal therapy j | 44 (71) | 11 (40.7) | 33 (94.3) | <.001* |
| Micafungin | 16 (25.8) | 1 (3.7) | 15 (42.6) | <.001* |
| Caspofungin | 14 (22.6) | 4 (14.8) | 10 (28.6) | .235 |
| Amphotericin B | 13 (21) | 0 | 13 (37.1) | ‐ |
| Anidulafungin | 9 (14.5) | 4 (14.8) | 5 (14.3) | >.99 |
| Voriconazole | 8 (12.9) | 2 (7.4) | 6 (17.1) | .447 |
| Isavuconazole | 3 (4.8) | 2 (7.4) | 1 (2.9) | .575 |
| Fluconazole | 3 (4.8) | 0 | 3 (8.6) | ‐ |
| Clinical outcome | TC ( n = 81) a | CANC cases (n = 27) b | CAC cases (n = 34) b | p value |
|---|---|---|---|---|
| Survived | 45 (55.6) | 21 (77.8) | 12 (35.3) | .002* |
| Death | 36 (44.4) | 6 (22.2) | 22 (64.7) |
The values in the table are expressed in numbers (n) and percentages (%). * ‘p’ values <.05 were considered significant.
Abbreviations: ARDS, Acute Respiratory Distress Syndrome; CAC, Candida auris candidemia; CANC, Candida auris non‐candidemia/colonised; TC, total cohort.
The data were extracted from the 11 studies which fulfilled inclusion criteria (TC). 13 , 14 , 15 , 16 , 22 , 23 , 24 , 25 , 26 , 27 , 28
Age range, male/female ratio, details of clinical specimens, underlying disease, iatrogenic risk factors, antifungal therapy and clinical outcome of CANC and CAC cases were extracted from 10 studies. 13 , 14 , 16 , 22 , 23 , 24 , 25 , 26 , 27 , 28
Mean age and mean days from first isolation of C. auris from admission for CANC and CAC cases were extracted from 9 studies. 13 , 14 , 15 , 16 , 23 , 24 , 25 , 27 , 28
Renal diseases were chronic kidney disease (CKD) and acute kidney injury (AKI). [TC: CKD (n = 9), and AKI (n = 2); CANC cases: CKD (n = 2); CAC cases: CKD (n = 4) and AKI (n = 2)].
Heart diseases were ischemic heart disease (IHD) and coronary heart disease (CAD). [TC: IHD (n = 5), and CAD (n = 3); CANC cases: IHD (n = 2), and CAD (n = 1); CAC cases: IHD (n = 2) and CAD (n = 2)].
Liver and Biliary diseases were chronic liver disease (CLD) and biliary lithiasis (BL). [TC: CLD (n = 3), and BL (n = 1); CANC cases: BL (n = 1); CAC cases: CLD (n = 3)].
Thromboembolic diseases were pulmonary embolism (PE) and deep venous thrombosis (DVT). [TC: PE (n = 3), and DVT (n = 2); CANC cases: PE (n = 1), and DVT (n = 2); CAC cases: PE (n = 2)].
Miscellaneous respiratory diseases were asthma, chronic obstructive pulmonary disease (COPD), respiratory failure (RF) and pneumothorax (PT). [TC: asthma (n = 4), COPD (n = 3), RF (n = 1), and PT (n = 1); CANC cases: asthma (n = 1), COPD (n = 2), RF (n = 1), and PT (n = 1); CAC cases: asthma (n = 3), and COPD (n = 1)].
Other risk factors included wound infections (WI), hyperlipidaemia (HLD), hypothyroidism (HT), stroke, dementia, stem cell transplant (SCT), systemic lupus erythematosus (SLE). [TC: WI (n = 6), HLD (n = 3), HT (n = 2), stroke (n = 1), dementia (n = 1), SCT (n = 1), and SLE (n = 1); CANC cases: WI (n = 2), HLD (n = 1), HT (n = 1), stroke (n = 1), dementia (n = 1), and SLE (n = 1); CAC cases: HLD (n = 2), HT (n = 1), and SCT (n = 1).
In patients with antifungal therapy, many patients received combination of antifungals for treatment.
FIGURE 3.
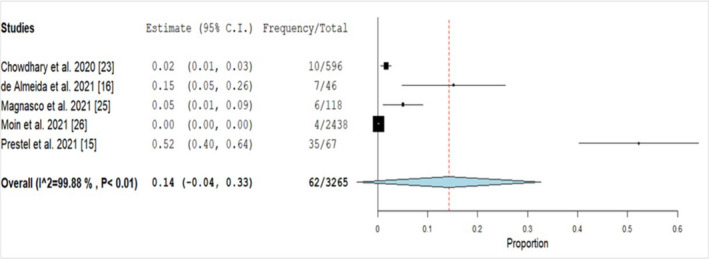
Forest plot of pooled prevalence of Candida auris infections in COVID‐19 patients. "Frequency" denotes total number of C. auris cases and "Total" denotes total number of COVID‐19 infected patients. References are given in square brackets. Abbreviations: C.I, Confidence Interval
3.1. Clinical specimens, isolation and identification of Candida auris
Of those 97 C. auris cases identified, 62 (64%) patients had details of clinical specimens from which C. auris was isolated. Majority of C. auris isolation was from blood (n = 35, 56.5%), followed by urine (n = 12, 19.4%), and respiratory specimens (n = 10, 16.1%) (Table 1). The identification of C. auris in seven studies was performed using matrix‐assisted laser desorption ionization time of flight mass spectrometry (MALDI‐TOF MS); four studies additionally used DNA sequencing of the internal transcribed spacer (ITS) region for confirmation. Whereas one study used API 20C AUX system (bioMerieux) and phenotypic methods for identification, and in three studies, method of identification is not mentioned.
As per the Center for Disease Control (CDC) (https://ndc.services.cdc.gov/case‐definitions/candida‐auris‐2019/), the case definition of C. auris infections/colonisation was specimens collected from invasive infections (eg blood, cerebrospinal fluid) were designated as confirmed cases, whereas isolation of C. auris from non‐invasive sites (wound swabs, urine and the respiratory tract) may reflect colonisation and not true infection. 29 Based on the definition, in the present study, C. auris cases (n = 62) were grouped into the following: (a) C. auris candidemia (CAC) cases (n = 35, 56.5%), (b) C. auris non‐candidemia/colonised (CANC) cases (n = 27, 43.5%; Table 1). The mean days from admission to the first isolation of C. auris in CANC and CAC cases were 27.7 and 22.8 days, respectively (Table 1).
3.2. Underlying disease and risk factors
Table 1 shows the underlying diseases associated with C. auris infections in COVID‐19 patients: diabetes mellitus (n = 35, 42.7%), hypertension (n = 27, 32.9%, obesity (n = 12, 14.6%), COVID‐19 associated acute respiratory distress syndrome (ARDS; n = 9, 11%) and malignancy (n = 8, 13.4%). Approximately 12% of the patients had no underlying disease. Further, CANC and CAC cases showed no significant differences with any underlying diseases analysed (Table 1). The pooled estimates of the underlying disease and iatrogenic risk factors of C. auris infections in COVID‐19 patients are depicted in Table 2.
TABLE 2.
Pooled estimates of underlying diseases and iatrogenic risk factors of Candida auris infections in COVID‐19 patients
| Underlying diseases | Total cohort a | Candida auris non‐Candidemia (CANC) b | Candida auris Candidemia (CAC) b | |||
|---|---|---|---|---|---|---|
| Estimate (95% CI) | I2 | Estimate (95% CI) | I2 | Estimate (95% CI) | I2 | |
| Diabetes Mellitus | 0.38 (0.21–0.51) | 59.32 | 0.16 (0.06‐0.25) | 22.76 | 0.17 (0.07‐0.27) | 33.81 |
| Hypertension | 0.33 (0.16–0.5) | 77.67 | 0.31 (0.15‐0.47) | 0 | 0.41 (0.22 −0.6) | 41.13 |
| Obesity | 0.16 (0.03–0.3) | 78.73 | 0.11 (0.04–0.18) | 0 | 0.07 (0.01‐0.13) | 0 |
| Immunocompetent | 0.09 (0.03–0.15) | 0.01 | 0.07 (0.08–0.12) | 0 | 0.06 (0.01–0.12) | 0.01 |
| COVID‐19 associated acute respiratory distress syndrome (ARDS) | 0.23(0.04‐0.42) | 93.6 | 0.07 (0.02‐0.12) | 0.06 | 0.06 (0.01‐0.12) | 0.03 |
| Malignancy | 0.09 (0.03‐0.14) | 0 | 0.07 (0.01‐0.13) | 0 | 0.07 (0.01‐0.12) | 0 |
| Renal diseases c | 0.12 (0.05–0.19) | 0 | 0.06 (0.01–0.11) | 0 | 0.1 (0.03–0.17) | 0 |
| Heart diseases c | 0.08 (0.03–0.14) | 0 | 0.07 (0.01–0.13) | 0 | 0.08 (0.02‐0.14) | 0 |
| Liver and biliary disease c | 0.05 (0.01–0.09) | 0 | 0.06 (0–0.11) | 0 | 0.07 (0.01–0.12) | 0 |
| Thromboembolic disease c | 0.05 (0.01–0.1) | 0 | 0.07 (0.01–0.13) | 0 | 0.07 ( 0.01–0.13) | 0 |
| Miscellaneous Respiratory Diseases c | 0.1 (0.03‐0.16) | 16.11 | 0.09 (0.02‐0.16) | 0 | 0.07 (0.01‐0.13) | 0 |
| Others c | 0.16 (0.07–0.24) | 36.39 | 0.08 (0.02–0.15) | 0 | 0.09 (0.02 −0.15) | 0.02 |
| Iatrogenic risk factors | ||||||
| Central venous catheter | 0.74 (0.56–0.93) | 88.54 | 0.33 (0.11–0.55) | 89.79 | 0.47 (0.24–0.71) | 88.07 |
| Intensive Care Unit (ICU) stay | 0.81 (0.62–1) | 94.08 | 0.43 (0.22–0.65) | 83.6 | 0.54 (0.32–0.75) | 80.94 |
| Broad spectrum antibiotics | 0.8 (0.61–1) | 93.83 | 0.41 (0.19–0.63) | 84.77 | 0.59 (0.35–0.79) | 83.6 |
| Mechanical ventilation | 0.69 (0.5–0.87) | 84.45 | 0.34 (0.15–0.53) | 77.44 | 0.38 (0.21–0.56) | 69.15 |
| Steroid therapy | 0.68 (0.46–0.91) | 93.94 | 0.39 (0.16–0.61) | 87.09 | 0.43 (0.23–0.64) | 77.47 |
| Urinary catheter | 0.54 (0.3–0.8) | 94.2 | 0.29 (0.09–0.5) | 86.22 | 0.32 (0.1–0.55) | 90.62 |
| Co‐infections along with C. auris | 0.58 (0.36–0.8) | 88.64 | 0.18 (0.06 −0.29) | 48.99 | 0.45 (0.25–0.65) | 77.93 |
| Previous antifungal therapy | 0.36 (0.13–0.59) | 94.87 | 0.24 (0.05–0.43) | 85.78 | 0.2 (0.02–0.38) | 86.14 |
| Haemodialysis | 0.07 (0.02–0.12) | 8.8 | 0.06 (0.01–0.11) | 0 | 0.07 (0.01–0.13) | 0 |
Abbreviations: ARDS, acute respiratory distress syndrome; CAC, Candida auris candidemia; CANC, Candida auris non‐candidemia/colonised; CI, confidence interval.
The data were extracted from the 11 studies which fulfilled inclusion criteria (Total cohort). 13 , 14 , 15 , 16 , 22 , 23 , 24 , 25 , 26 , 27 , 28 The denominators used in the analysis for the total cohort are n = 82.
The data for underlying diseases and iatrogenic risk factors of CANC and CAC cases were extracted from 10 studies. 13 , 14 , 16 , 22 , 23 , 24 , 25 , 26 , 27 , 28 Denominators used in the analysis for comparison of CANC and CAC group is n = 62.
Refer to Table 1 for the different risk factors placed under the subheadings, such as renal, heart, liver, thromboembolic, miscellaneous respiratory disease and other risk factors.
Similar to underlying diseases, iatrogenic risk factors such as central venous catheter (n = 63, 76.8%), intensive care unit (ICU) stay (n = 62, 75.6%), broad‐spectrum antibiotic usage (n = 61, 74.3%), mechanical ventilation (n = 57, 69.5%), steroid therapy (n = 51, 62.2%) and urinary catheter (n = 47, 57.3%) showed no significance differences between the CANC and CAC groups. (Table 1). The pooled estimates of co‐infections in C. auris among the total cohort of COVID‐19 patients was 58% (with high heterogeneity I2 = 88.65) (Table 2).
3.3. Antifungal therapy and outcome of Candida auris infections
A total of 44 patients received antifungal therapy (Table 1); echinocandins (n = 33, 75%) were commonly used, followed by amphotericin B (n = 13, 29.6%). No significant difference was observed between different antifungal medications and the survival (data not shown). The mortality rate of the total cohort (n = 81) was at 44.44% (Table 1), in comparison with CANC cases was at 22.2% (n = 6) and CAC case was at 64.7% (n = 22) (p = <.002). The pooled survival estimates of 61 patients in CANC and CAC groups were 37% and 16%, with low heterogeneity (I2 = 40.36 and 0%), respectively (Figure 4). The mortality rate in patients with underlying disease and iatrogenic risk factors such as diabetes mellitus, central venous catheter, ICU stay, broad‐spectrum antibiotic usage, mechanical ventilation, steroid therapy and urinary catheter were significantly associated with a higher mortality rate in the CAC group compared with the CANC group (Table 3).
FIGURE 4.
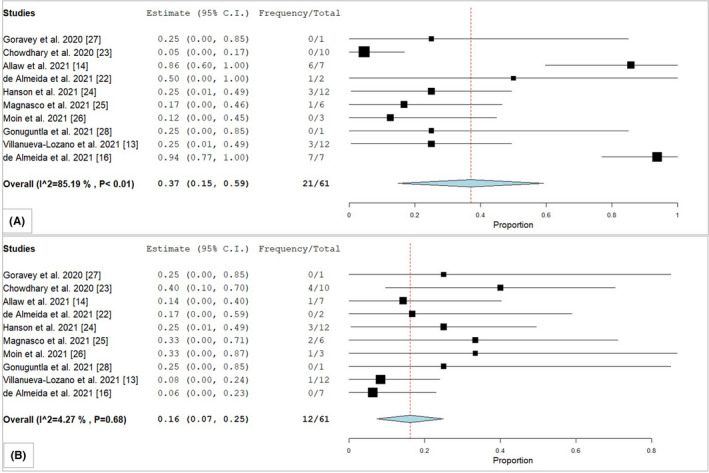
Forest plot of pooled survival estimates of (A) Candida auris non‐candidemia/colonised (CANC) and (B) Candida auris candidemia (CAC) cases in COVID‐19 patients. "Frequency" denotes total number of patients survived with C. auris infections and "Total" denotes total number of C. auris cases reported in each study. References are given in square brackets. Abbreviations: C.I, Confidence Interval
TABLE 3.
Underlying disease and iatrogenic risk factors associated with mortality in Candida auris non‐candidemia/colonised (CANC) and Candida auris candidemia (CAC) cases
| Underlying disease a and Iatrogenic risk factors | Candida auris non‐candidemia (CANC) b (n) | Candida auris candidemia (CAC) b (n) | Death in CANC group (n) | Death in CAC group (n) | p value |
|---|---|---|---|---|---|
| Diabetes mellitus | 11 | 12 | 2 | 9 | .012* |
| Hypertension | 10 | 17 | 3 | 12 | .056 |
| Central venous catheter | 19 | 27 | 3 | 18 | .0009* |
| Intensive care unit (ICU) stay | 27 | 33 | 6 | 22 | .0008* |
| Broad spectrum antibiotics | 26 | 34 | 5 | 22 | .0006* |
| Mechanical ventilation | 22 | 24 | 5 | 18 | .0009* |
| Steroid therapy | 24 | 27 | 5 | 20 | .0002* |
| Urinary catheter | 17 | 19 | 3 | 13 | .0031* |
| Co‐infections along with C. auris | 13 | 20 | 5 | 15 | .067 |
| Previous antifungal therapy | 12 | 7 | 0 | 4 | .009* |
The values in the table are expressed in numbers (n). ‘n’ denotes the total number of patients. * ‘p’ values <.05 were considered significant.
Abbreviations: CAC, Candida auris candidemia; CANC, Candida auris non‐candidemia/colonised.
Underlying disease and mortality association was statistically analysed for diabetes mellitus and hypertension alone. The number of cases for in other underlying diseases were less (refer Table 1), hence no statistical analysis was performed.
3.4. Antifungal susceptibility of Candida auris isolates from COVID‐19 patients
Table 4 shows the antifungal susceptibility testing of C. auris isolates (n = 41). Of those tested isolates, resistance was noted in 33 isolates (80.5%) to fluconazole (MIC ≥ 32 mg/L), followed by 19 (46.3%) to amphotericin B (MIC ≥ 2 mg/L), 5 (12.8%) to caspofungin (MIC ≥ 2 mg/L), 2 (5.1%) to anidulafungin (MIC ≥ 4 mg/L), 1 (3.7%) to micafungin (MIC ≥ 4 mg/L), and 7 (43.8%) to 5‐flucytosine (MIC ≥ 32 mg/L). Voriconazole non‐susceptibility (MIC ≥ 2 mg/L) was observed in 12 (29.3%) C. auris isolates (Table 4).
TABLE 4.
Antifungals minimum inhibitory concertation (MICs) for Candida auris isolates from COVID‐19 patients
| Antifungals | No. of isolates | MICs in Range (mg/L) | Geometric Mean | MIC50 | MIC90 | No. of C. auris isolates with MICs (mg/L) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.015 | 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 0.75 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | ||||||
| Amphotericin B | 41 | 0.125–8 | 1.29 | 1 | 4 | 0 | 0 | 0 | 1 | 1 | 8 | 5 | 7 | 9 | 9 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Caspofungin | 39 | 0.125–8 | 0.597 | 0.5 | 2 | 0 | 0 | 0 | 5 | 7 | 8 | 0 | 14 | 3 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Micafungin | 27 | 0.03–8 | 0.207 | 0.25 | 1 | 0 | 1 | 4 | 8 | 8 | 3 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Anidulafungin | 38 | 0.06–8 | 0.431 | 0.5 | 1 | 0 | 0 | 2 | 5 | 8 | 12 | 0 | 9 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fluconazole | 41 | 2–512 | 92.83 | 256 | 256 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 4 | 5 | 4 | 1 | 19 | 4 |
| Voriconazole | 41 | 0.03–8 | 0.56 | 1 | 2 | 0 | 3 | 1 | 5 | 6 | 4 | 0 | 10 | 9 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Isavuconazole | 32 | 0.03–1 | 0.15 | 0.125 | 0.5 | 0 | 3 | 6 | 11 | 5 | 6 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Posaconazole | 35 | 0.015–8 | 0.095 | 0.125 | 0.25 | 1 | 9 | 5 | 14 | 3 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Itraconazole | 23 | 0.06–16 | 0.38 | 0.25 | 1 | 0 | 0 | 2 | 2 | 9 | 6 | 0 | 1 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| 5‐Flucytosine | 16 | 0.25‐–64 | 6.44 | 8 | 64 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 2 | 0 | 0 | 2 | 1 | 1 | 6 | 0 | 0 | 0 |
Furthermore, 8 (19.5%) isolates were resistant to fluconazole and voriconazole. Multidrug resistance (resistance to two different classes of antifungal drugs) was noted in 22 (53.6%) C. auris isolates; of those, 18 (81.8%) and 4 (18.2%) isolates were resistant to two and three classes of antifungal drugs, respectively. Amphotericin B plus azole resistance was noted in 10 (45.5%), followed by echinocandins and azole (n = 4, 18.2%), azole and 5‐flucytosine (n = 3, 13.6%), amphotericin B and echinocandins (n = 1, 4.6%), amphotericin B, azole and 5‐flucytosine (n = 3, 13.6%) and echinocandins, azole and 5‐flucytosine (n = 1, 4.6%).
4. DISCUSSION
The present systematic review analysed the published cases of C. auris in COVID‐19 patients, providing insight on prevalence, underlying disease/risk factors, treatment and disease outcome. The review was conducted per PRISMA guidelines, including only the articles that met the study criteria. This review has provided an update on the epidemiology of C. auris infections in COVID‐19 patients.
In the present study, majority of the C. auris‐infected patients were adults (99%), with one case in the paediatric population, similar to previous reports. 4 , 6 , 10 In this study, the pooled prevalence of C. auris infections in COVID‐19 patients was 14%. The true prevalence of C. auris infections in the global population is largely unknown. As per multiple studies, the prevalence of C. auris in candidemia patients (non‐COVID‐19 cases) ranges between 5‐30%. 30 Further, many C. auris colonisation patients have also been identified in countries like the USA, Europe, India, South Africa and henceforth. 5 , 31 , 32 , 33
As per CDC recommendation on C. auris definition for colonisation (non‐invasive sites) and true infections (invasive sites), 29 in the present study, the cases were classified as C. auris non‐candidemia/colonised (CANC) and C. auris candidemia (CAC) cases, respectively. A total of 35 (56.5%) CAC cases and 27 (43.5%) CANC cases were identified. The underlying disease/iatrogenic risk factors in identified both groups were: hypertension (43.5%), diabetes mellitus (37%), obesity (19.4%), COVID‐19‐associated ARDS (14.5%) and renal diseases (13%); whereas the iatrogenic risk factors included broad‐spectrum antibiotic usage (98.4%), ICU stay (97%), steroid therapy (82.3%), the central venous catheter (75.8%), mechanical ventilation (74.2%) and steroid therapy (82.2%). The respective analysis of C. auris and COVID‐19 patients showed that both groups shared the underlying disease mentioned above. 3 , 4 , 9 , 10 , 11 , 12 Multiple studies have reported central venous catheter, previous antifungal exposure, mechanical ventilation, and prolonged ICU stay as significant risk factors for C. auris colonisation/infection. 7 , 34 , 35 Thus, the ongoing COVID‐19 pandemic would become a perfect battlefield for outbreaks of C. auris because of the similar underlying diseases and the risk factors, which increases the chance of C. auris infections in COVID‐19 patients. Further, a recent study from India confirmed these findings, that the treatment with interleukin‐6 antagonists such as tocilizumab, prolonged ICU stay, mechanical ventilation and raised ferritin levels were identified as significant risk factors of candidemia (majority of reported cases in the study were due to C. auris) in COVID‐19 patients. 36
Furthermore, during the COVID‐19 pandemic, the existing ICU facilities have seen overflowing patients, with a high burden of patients, there was a challenge in implementing the infection control practices. Of the included studies in this review, a few studies documented the source for the C. auris outbreak, and the infection prevention and control (IPCs) measures to contain the C. auris infection in COVID‐19 patients. 13 , 14 , 16 Allaw et al. reported C. auris outbreak in Lebanon in a tertiary care centre for 13 weeks. Following the first case of C. auris infection, IPC practices such as hand hygiene using antiseptics, disinfection of floors and surfaces of patient's room, screening for C. auris in environmental samples and skin colonisation in patients, 4% chlorhexidine bath for C. auris skin decolonisation was initiated. C. auris was not isolated from environmental samples; however, of the 26 patients screened for skin colonisation, one patient grew C. auris. Authors attributed the delay in reporting and identifying the first case of C. auris to the outbreak, which delayed the implementation of IPC measures. 14 Villanueva‐Lozano et al. reported C. auris outbreak in COVID‐19 patients in Mexico, and the authors isolated three environmental isolates from their bedrooms. The phylogenetic analysis of the clinical and environmental isolates clustered together, suggesting close similarities among the isolates. 13 These findings show that the environmental contamination of hospitals remains a possibility either by cross‐contamination of the equipment or by the healthcare providers. However, Chowdhary et al. 23 proposed that C. auris infection in COVID‐19 patients may not be transmitted by healthcare personnel because of personal protective equipment (PPE). Further, the authors cautioned that improper use of PPE may lead to contamination and disease transmission. 23 A study from Brazil documented the source of C. auris outbreak in the hospital settings; the environmental screening showed C. auris contamination from auxiliary digital thermometers (17%), bed rails (15%), and intravenous infusion pumps (11%) and tray tables (11%). The study reported that C. auris colonisation of digital thermometer was the significant risk factor associated with C. auris colonisation in patients, which correlated with the high isolation rate of C. auris from axillae of the patients. 16 Similarly, Eyre et al. 7 reported that skin surface axillary temperature reusable probes were significantly associated with C. auris colonisation/infection (86% in the patient group versus 34% in controls). Proper surveillance for C. auris colonisation and implementation of infection control practices may stop the spread of C. auris infections/colonisation in COVID‐19 ICU settings. 7 , 37
Candida auris multidrug‐resistant isolates have been reported from Asia, the USA, Europe and Africa. 7 , 38 , 39 , 40 , 41 , 42 In the present study, C. auris isolates were resistant to fluconazole (81%), followed by voriconazole (29.3%), amphotericin B (46.3%), caspofungin (12.8%), anidulafungin (5.1%), micafungin (3.7%) and 5‐flucytosine (43.8%). Similarly, antifungal susceptibility testing of C. auris isolates from multiple countries reported a large number of isolates are resistant to fluconazole (35%–100%), followed by amphotericin B resistance (8%–61%), echinocandins resistance (0.5%–3%) and voriconazole (14%–90%). 7 , 38 , 39 , 40 , 41 In the present study, multidrug resistance was noted in 53.6% of C. auris isolates, similar to the previously reported studies (6%–61%). 40 , 41 , 42
In the present study, 44 (71%) patients from CANC (n = 11, 25%) and CAC (n = 33, 75%) group received antifungal therapy. Globally, C. auris isolates exhibit a higher susceptible pattern to echinocandins, 7 , 38 , 39 , 40 , 41 making them the drug of choice for C. auris infections. Similarly, echinocandins (75%) were the most common drug used in this study. The survival rate for CANC and CAC groups was 77.8% and 35.3%, respectively (p < .002). Multiple systematic reviews showed that mortality in C. auris cases was at 39% to 47.5%. 4 , 43 Similar to the present study's findings, Sayeed et al. 10 reported a survival rate of 54% in C. auris candidemia cases and 67% in colonised cases. Further, this study showed that iatrogenic risk factors such as prolonged ICU stay, mechanical ventilation, steroid therapy, broad‐spectrum antibiotics and central venous catheters were significantly associated with high mortality in CAC patients compared with the CANC group. These findings caution that healthcare personnel should be vigilant while treating severe COVID‐19 patients, as they are more vulnerable to C. auris infection. Despite the treatment with antifungals, a high mortality rate is seen in C. auris infections, making them a global threat.
5. CONCLUSION
The study highlights the role of hospital‐acquired C. auris infections in COVID‐19 patients. Despite the multiple risk factors possibly favouring the C. auris infections in COVID‐19 patients, the prevalence of the disease remains unchanged compared with the pre‐pandemic. However, one must be cautioned that COVID‐19 patients may be more vulnerable to C. auris infections because of the overlapping risk factors. Increased burden of colonised patients with C. auris in ICU settings may lead to person‐to‐person transmission. Hence, proper infection control practices and strict hospital surveillance for screening and isolating C. auris colonised patients in the COVID‐19 ICUs may prevent the potential outbreaks in hospital settings.
CONFLICT OF INTEREST
The authors declare no competing interest.
AUTHOR CONTRIBUTIONS
HPP conceptualised the idea of this study. HPP, KSV and KCP designed the study. HPP and KSV did primary data extraction for the study and wrote the manuscript. KCP and HPP performed the analysis. The final draft of the manuscript was corrected and proofread by all the authors.
Vinayagamoorthy K, Pentapati KC, Prakash H. Prevalence, risk factors, treatment and outcome of multidrug resistance Candida auris infections in Coronavirus disease (COVID‐19) patients: A systematic review. Mycoses. 2022;65:613–624. doi: 10.1111/myc.13447
DATA AVAILABILITY STATEMENT
The data generated in the study was provided in the tables and figures of the manuscript.
REFERENCES
- 1. Nori P, Cowman K, Chen V, et al. Bacterial and fungal coinfections in COVID‐19 patients hospitalized during the New York City pandemic surge. Infect Control Hosp Epidemiol. 2021;42(1):84‐88. doi: 10.1017/ice.2020.368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang S, Hua M, Liu X, et al. Bacterial and fungal co‐infections among COVID‐19 patients in intensive care unit. Microbes Infect. 2021;23(4–5):104806. doi: 10.1016/j.micinf.2021.104806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Al‐Rashdi A, Al‐Maani A, Al‐Wahaibi A, Alqayoudhi A, Al‐Jardani A, Al‐Abri S. Characteristics, risk factors, and survival analysis of Candida auris cases: results of one‐year national surveillance data from Oman. J Fungi. 2021;7(1):31. doi: 10.3390/jof7010031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hu S, Zhu F, Jiang W, et al. Retrospective analysis of the clinical characteristics of Candida auris infection worldwide from 2009 to 2020. Front Microbiol. 2021;12:1‐9. doi: 10.3389/fmicb.2021.658329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sabino R, Veríssimo C, Pereira ÁA, Antunes F Candida auris, an agent of hospital‐associated outbreaks: which challenging issues do we need to have in mind? Microorganisms. 2020;8(2):181. doi: 10.3390/microorganisms8020181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rudramurthy SM, Chakrabarti A, Paul RA, et al. Candida auris candidaemia in Indian ICUs: analysis of risk factors. J Antimicrob Chemother. 2017;72(6):1794‐1801. doi: 10.1093/jac/dkx034 [DOI] [PubMed] [Google Scholar]
- 7. Eyre DW, Sheppard AE, Madder H, et al. A Candida auris outbreak and its control in an intensive care setting. N Engl J Med. 2018;379(14):1322‐1331. doi: 10.1056/NEJMoa1714373 [DOI] [PubMed] [Google Scholar]
- 8. Pfaller MA, Messer SA, Deshpande LM, Rhomberg PR, Utt EA, Castanheira M. Evaluation of synergistic activity of Isavuconazole or voriconazole plus anidulafungin and the occurrence and genetic characterization of Candida auris detected in a surveillance program. Antimicrob Agents Chemother. 2021;65(4):6‐10. doi: 10.1128/AAC.02031-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Osei SJ. Candida auris: A systematic review and meta‐analysis of current updates on an emerging multidrug‐resistant pathogen. Microbiologyopen. 2018;7(4):e00578. doi: 10.1002/mbo3.578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sayeed MA, Farooqi J, Jabeen K, Awan S, Mahmood SF. Clinical spectrum and factors impacting outcome of Candida auris: a single center study from Pakistan. BMC Infect Dis. 2019;19(1):384. doi: 10.1186/s12879-019-3999-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rosenthal N, Cao Z, Gundrum J, Sianis J, Safo S. Risk factors associated with in‐hospital mortality in a US National sample of patients with COVID‐19. JAMA Netw Open. 2020;3(12):e2029058. doi: 10.1001/jamanetworkopen.2020.29058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chidambaram V, Tun NL, Haque WZ, et al. Factors associated with disease severity and mortality among patients with COVID‐19: a systematic review and meta‐analysis. PLoS One. 2020;15(11):e0241541. doi: 10.1371/journal.pone.0241541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Villanueva‐Lozano H, Treviño‐Rangel RJ, González GM, et al. Outbreak of Candida auris infection in a COVID‐19 hospital in Mexico. Clin Microbiol Infect. 2021;27(5):813‐816. doi: 10.1016/j.cmi.2020.12.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Allaw F, Kara Zahreddine N, Ibrahim A, et al. First Candida auris outbreak during a COVID‐19 pandemic in a tertiary‐care center in Lebanon. Pathogens. 2021;10(2):1‐10. doi: 10.3390/pathogens10020157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Prestel C, Anderson E, Forsberg K, et al. Candida auris outbreak in a COVID‐19 specialty care unit ‐ Florida, July‐August 2020. MMWR Morb Mortal Wkly Rep. 2021;70(2):56‐57. doi: 10.15585/mmwr.mm7002e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nobrega de Almeida J, Brandão IB, Francisco EC, et al. Axillary digital thermometers uplifted a multidrug‐susceptible Candida auris outbreak among COVID‐19 patients in Brazil. Mycoses. 2021;64(9):1062‐1072. doi: 10.1111/myc.13320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan‐a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Whiting P, Rutjes AWS, Reitsma JB, Bossuyt PMM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25. doi: 10.1186/1471-2288-3-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Munn Z, Barker TH, Moola S, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 2020;18(10):2127‐2133. doi: 10.11124/JBISRIR-D-19-00099 [DOI] [PubMed] [Google Scholar]
- 20. Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the gap between methodologists and end‐users: R as a computational back‐end. J Stat Softw. 2012;49(5):1‐15. doi: 10.18637/jss.v049.i05 [DOI] [Google Scholar]
- 21. Viechtbauer W. Conducting meta‐analyses in R with the metafor Package. J Stat Softw. 2010;36(3):1‐48. doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 22. de Almeida JN, Francisco EC, Hagen F, et al. Emergence of Candida auris in Brazil in a COVID‐19 intensive care unit. J Fungi. 2021;7(3):220. doi: 10.3390/jof7030220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chowdhary A, Tarai B, Singh A, Sharma A. Multidrug‐resistant Candida auris infections in critically ill Coronavirus disease patients, India, April‐July 2020. Emerg Infect Dis. 2020;26(11):2694‐2696. doi: 10.3201/eid2611.203504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hanson BM, Dinh AQ, Tran TT, et al. Candida auris invasive infections during a COVID‐19 case surge. Antimicrob Agents Chemother. 2021;65(10):e0114621. doi: 10.1128/AAC.01146-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Magnasco L, Mikulska M, Giacobbe DR, et al. Spread of carbapenem‐resistant gram‐negatives and Candida auris during the COVID‐19 pandemic in critically ill patients: one step back in antimicrobial stewardship? Microorganisms. 2021;9(1):95. doi: 10.3390/microorganisms9010095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moin S, Farooqi J, Rattani S, Nasir N, Zaka S, Jabeen K C. auris and non‐C auris candidemia in hospitalized adult and pediatric COVID‐19 patients; single center data from Pakistan. Med Mycol. 2021;59(12):1238‐1242. doi: 10.1093/mmy/myab057 [DOI] [PubMed] [Google Scholar]
- 27. Goravey W, Ali GA, Ali M, Ibrahim EB, Al Maslamani M, Abdel HH. Ominous combination: COVID‐19 disease and Candida auris fungemia—Case report and review of the literature. Clin Case Rep. 2021;9(9):1‐9. doi: 10.1002/ccr3.4827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tej GV, Britney C, Susan L. A case of Hafnia alvei and Candida auris bloodstream infection in patients with COVID‐19. Chest. 2021;160(4):A1728. doi: 10.1016/j.chest.2021.07.1573 [DOI] [Google Scholar]
- 29. Council of State and Territorial Epidemiologists . Standardized case definition for Candida auris causing clinical infection and colonization in people, (17th‐ID‐03). Atlanta, GA: Council of State and Territorial Epidemiologists; 2017. Accessed February 01, 2022. https://www.chicagohan.org/documents/14171/427345/CSTE+case+definition.pdf/736d8b57‐1084‐cdd4‐ed66‐d39ae1cc4356?t=1611162106400
- 30. Cortegiani A, Misseri G, Fasciana T, Giammanco A, Giarratano A, Chowdhary A. Epidemiology, clinical characteristics, resistance, and treatment of infections by Candida auris . J Intensive Care. 2018;6(1):69. doi: 10.1186/s40560-018-0342-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yadav A, Singh A, Wang Y, et al. Colonisation and transmission dynamics of Candida auris among chronic respiratory diseases patients hospitalised in a chest hospital, Delhi, India: a comparative analysis of whole genome sequencing and microsatellite typing. J Fungi. 2021;7(2):81. doi: 10.3390/jof7020081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Govender NP, Magobo RE, Mpembe R, et al. Candida auris in South Africa, 2012–2016. Emerg Infect Dis. 2018;24(11):2036‐2040. doi: 10.3201/eid2411.18-0368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Biswal M, Rudramurthy SM, Jain N, et al. Controlling a possible outbreak of Candida auris infection: lessons learnt from multiple interventions. J Hosp Infect. 2017;97(4):363‐370. doi: 10.1016/j.jhin.2017.09.009 [DOI] [PubMed] [Google Scholar]
- 34. Rossow J, Ostrowsky B, Adams E, et al. Factors associated with Candida auris colonization and transmission in skilled nursing facilities with ventilator units, New York, 2016–2018. Clin Infect Dis. 2021;72(11):e753‐e760. doi: 10.1093/cid/ciaa1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shastri PS, Shankarnarayan SA, Oberoi J, Rudramurthy SM, Wattal C, Chakrabarti A Candida auris candidaemia in an intensive care unit ‐ Prospective observational study to evaluate epidemiology, risk factors, and outcome. J Crit Care. 2020;57:42‐48. doi: 10.1016/j.jcrc.2020.01.004 [DOI] [PubMed] [Google Scholar]
- 36. Rajni E, Singh A, Tarai B, et al. A high frequency of Candida auris blood stream infections in Coronavirus disease 2019 patients admitted to intensive care units, Northwestern India: a case control study. Open Forum Infect Dis. 2021;8(12):ofab452. doi: 10.1093/ofid/ofab452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Umamaheshwari S, Neelambike SM, Shankarnarayan SA, et al. Clinical profile, antifungal susceptibility, and molecular characterization of Candida auris isolated from patients in a South Indian surgical ICU. J Mycol Med. 2021;31(4):101176. doi: 10.1016/j.mycmed.2021.101176 [DOI] [PubMed] [Google Scholar]
- 38. Chowdhary A, Prakash A, Sharma C, et al. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009–17) in India: role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J Antimicrob Chemother. 2018;73(4):891‐899. doi: 10.1093/jac/dkx480 [DOI] [PubMed] [Google Scholar]
- 39. Escandón P, Cáceres DH, Lizarazo D, Lockhart SR, Lyman M, Duarte C. Laboratory‐based surveillance of Candida auris in Colombia, 2016–2020. Mycoses. 2022;65(2):222‐225. doi: 10.1111/myc.13390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maphanga TG, Naicker SD, Kwenda S, et al. In vitro antifungal resistance of Candida auris isolates from bloodstream infections, South Africa. Antimicrob Agents Chemother. 2021;65(9):e0051721. doi: 10.1128/AAC.00517-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhu Y, O’Brien B, Leach L, et al. Laboratory analysis of an outbreak of Candida auris in New York from 2016 to 2018: impact and lessons learned. J Clin Microbiol. 2020;58(4):e01503. doi: 10.1128/JCM.01503-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lockhart SR, Etienne KA, Vallabhaneni S, et al. Simultaneous emergence of multidrug‐resistant Candida auris on 3 continents confirmed by whole‐genome sequencing and epidemiological analyses. Clin Infect Dis. 2017;64(2):134‐140. doi: 10.1093/cid/ciw691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen J, Tian S, Han X, et al. Is the superbug fungus really so scary? A systematic review and meta‐analysis of global epidemiology and mortality of Candida auris . BMC Infect Dis. 2020;20(1):827. doi: 10.1186/s12879-020-05543-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated in the study was provided in the tables and figures of the manuscript.


