Abstract
Background
Candida auris a frequently multidrug‐resistant yeast species that poses a global health threat due to its high potential for hospital outbreaks. While C. auris has become endemic in parts of Asia and Africa, transmissions have so far rarely been reported in Western Europe except for Great Britain and Spain. We describe the first documented patient‐to‐patient transmission of C. auris in Germany in a COVID‐19 intensive care unit (ICU) and infection control measures implemented to prevent further spread of the pathogen.
Methods
Identification of C. auris was performed by MALDI‐TOF and confirmed by internal transcribed spacer (ITS) sequencing. Antifungal susceptibility testing was carried out. We conducted repeated cross‐sectional examinations for the presence of C. auris in the patients of the affected ICU and investigated possible routes of transmission.
Results
The index patient had been transferred to Germany from a hospital in Northern Africa and was found to be colonised with C. auris. The contact patient developed C. auris sepsis. Infection prevention and control (IPC) measures included strict isolation of the two C. auris patients and regular screening of non‐affected patients. No further case occurred during the subsequent weeks. Reusable blades used in video laryngoscope‐guided intubation were considered as the most likely vehicle of transmission.
Conclusions
In view of its high risk of transmission, vigilance regarding C. auris colonisation in patients referred from endemic countries is crucial. Strict and immediate IPC measures may have the potential to prevent C. auris outbreaks.
Keywords: antifungal treatment, Candida auris, COVID‐19, infection prevention and control measures
1. INTRODUCTION
Candida auris an emerging yeast pathogen that is increasingly associated with hospital outbreaks worldwide. 1 , 2 , 3 Genomic epidemiology has suggested the simultaneous global emergence of several distinct clades. 4 Treatment of C. auris infections is hampered by the frequently reduced susceptibility to different classes of antifungal agents, including multi‐ and pan‐antifungal resistance. 5 In some regions, especially in Africa and Asia, C. auris has become an endemic pathogen. 6 In Europe, large outbreaks have been reported recently from intensive care units (ICUs) in the United Kingdom and Spain. 7 , 8 These outbreaks are often prolonged and difficult to contain. In Germany, only isolated cases of C. auris have been reported thus far. 9 Most of these cases have occurred in patients who had recently been in contact with hospitals / healthcare providers abroad. Although since the first published report the number of cases documented by the National Reference Center for Invasive Fungal Infections (NRZMyk) has increased to >25 (unpublished data), local transmission of the fungus has not yet been reported so far. 9 Since the onset of the COVID‐19 pandemic, an increased number of C. auris infections in COVID‐19 patients have been reported such as from India and United States. 10 Here, we describe the first documented transmission of C. auris in Germany between two critically ill COVID‐19 patients, actions taken for infection prevention and control (IPC) and the identification of the probable path of transmission.
2. METHODS
2.1. Setting
In response to the high demand during the SARS‐CoV‐2 pandemic, our university hospital with over 3000 patient beds established several temporary COVID‐19 ICUs. For this purpose, a building with a capacity of up to one hundred ICU beds on three levels was established. Each level is U‐shaped with two wings (wing A and B) hosting between 15 and 24 beds each, with one to four beds in each room. Staff were recruited from other ICUs, operating rooms and non‐intensive care units. The treatment spectrum included the full range of high‐level critical care, except ECMO (extracorporal membrane oxygenation) therapy, which was conducted only on other dedicated ICUs.
2.2. Ethics
As all patient data were anonymised for this report and microbiological diagnostics was clinical routine, ethics approval was not required according to §25 Berliner Landeskrankenhausgesetz (Berlin hospital law).
2.3. Laboratory methods
Specimens for routine fungal analysis were inoculated on Sabouraud Glucose Selective Agar with gentamicin and chloramphenicol (Thermo Scientific/ Oxoid PO5086A) and BBLTM CHROMagarTM Candida Medium (BD). Agar plates were incubated at 28°C and 37°C, respectively, for up to seven days. Samples, such as cerebrospinal fluids (CSF), biopsies, tissue samples or bronchoalveolar lavage fluids (BAL), were also inoculated on brain‐heart‐infusion slants (BHI) with or without (CSF samples) gentamicin and chloramphenicol (Sifin Diagnostics GmbH) and incubated at 28°C for four weeks. For specimens from sterile compartments like CSF, a supplemental Sabouraud liquid medium (bioMérieux) was used and incubated at 28°C for seven days. Specimens for patient screening and environmental swabs were inoculated on CHROMagar™ Candida Plus (CHROMagar) and incubated at 37°C for four days. Yeast isolates were identified with VITEK® MS v3.2.0 (bioMérieux), a system that uses Matrix‐Assisted Laser Desorption Ionization Time‐of‐Flight (MALDI‐TOF) technology. Susceptibility testing was done using VITEK® 2 System with AST Y08 cards and ETEST gradient technology (bioMérieux). The first C. auris isolate of each patient was sequenced using the MiSeqTM system (Illumina, Inc) with an Illumina Internal Transcribed Spacer (ITS) rRNA sequencing protocol and amplicon primers ITS1‐30F and ITS1‐217R as described elsewhere. 11 In the NRZMyk, species, identification (ID) was confirmed using ITS1/2 sequencing as described earlier. 12 Primer sequences ‘CACACGGGGAATATCTAACTTAGCA’ (forward) and ‘AGTACTTGCTCAGCTTCCACAGAG’ (reverse) were used for MAT‐ locus (Mating Type locus) amplification; ‘CACCAACAGAATGAACCTCGACAT’ vs. ‘GTAGTGACCCTTAGGCACAACATA’ and ‘TAGGCTGGAGCTGGTTTGGTAAGA’ vs. ‘GTGACATCGTTCCTGACAGGGA’ were used for ERG11 amplification. Oligonucleotide primers are shown in 5’–3’ direction. Broth microdilution (BM) was performed using EUCAST reference methodology. 13 FKS hotspot regions were performed as described earlier. 12
2.4. Investigation of possible routes of transmission
All procedures that had required patient transport and all measures involving the use of medical devices on both C. auris patients were extracted from the electronic patient file together with dates and times. All such records sorted by device were tabulated, and time associations of the index case and the contact patient were identified.
3. RESULTS
3.1. C. auris index case
A 65‐year‐old women with COVID‐19 was admitted from Northern Africa for intensive care. Prior treatment comprised remdesivir, tocilizumab, hydroxychloroquine and systemic steroids. The patient was put in single room isolation and 1:1 nursing care was initiated, since a carbapenem‐resistant Klebsiella pneumoniae (CRKP) colonisation was identified during admission screening. Daily antiseptic bathing with octenidine‐impregnated cloths was conducted, a procedure performed in all our ICUs with the aim of reducing hospital‐acquired bloodstream infections. 14 The day after admission mechanical ventilation and proning were initiated for adult respiratory distress syndrome (ARDS). In addition, voriconazole was initiated for radiographical signs of pulmonary aspergillosis. However, this antifungal therapy was stopped when a filamentous fungal infection was not confirmed. On Day 11 of her hospital stay, C. auris was found for the first time in a urine sample. On Day 27, C. auris was confirmed in bronchoalveolar lavage (BAL) obtained on Day 16 (Figure 1). Both findings were defined as colonisation, and no treatment was initiated. The already implemented IPC measures (single room isolation, 1:1 nursing care) were maintained. Because of limitations in efficacy of quaternary ammonium compounds (QAC) against C. auris, we changed from a QAC based standard to a peracetic acid (PAA) based regimen to disinfect patients’ rooms. 15 , 16 , 17 , 18 Similarly, we changed disinfection of ultrasound probes from QAC based wipes to one with hydrogen peroxide. Surface disinfection continued with alcohol‐based products, if the surface tolerated alcohol. Information material on C. auris and the implemented measures were prepared and distributed to the healthcare personnel.
FIGURE 1.
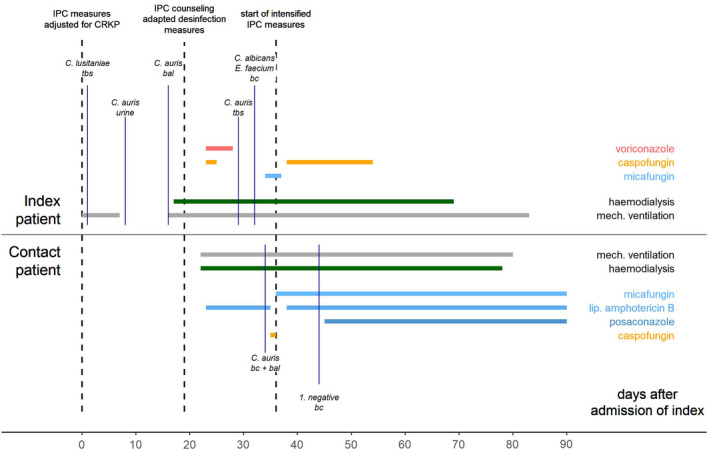
Clinical course of C. auris index and contact patients from Day 0 (admission of index patient) to Day 90 (transfer of index to rehabilitation clinic) with display of organ support, antifungal treatment and the timing of obtaining the most relevant microbiological samples with evidence of fungal infection/colonisation. Infection prevention and control (IPC) measures for suspected multidrug resistant bacterial colonisation were established immediately after admission of the index patient; tbs: tracheobronchial secretion, bc: blood culture, bal: bronchioalveolar lavage; C.: Candida; E.: Enterococcus; CRKP: carbapenem‐resistant Klebsiella pneumoniae; IPC: infection and prevention control
On Day 90, the index patient was discharged and transferred to a rehabilitation clinic, while still being colonised with C. auris (inguinal crease, throat, nose and urine).
3.2. Detection of C. auris transmission
Nine days after confirmation of C. auris colonisation of the index patient (Day 27 of her stay), the fungus was detected in the tracheobronchial secretion (TBS) and blood culture of another patient in the same ward. The contact patient was a 60‐year‐old male admitted 15 days after the index patient. His past medical history included a lung transplant for exogenous allergic alveolitis in 2018 and moderate chronic kidney disease (glomerular filtration rate approximately 50 ml/min). He had been referred from an external German hospital under oxygen insufflation and was intubated on Day 9 of his inpatient stay for respiratory exhaustion. He was located in a room approx. 12 metres away from the room where the index patient was treated. On Day 20 after admission, the patient developed severe sepsis. Blood cultures were obtained and revealed C. auris three days later. C. auris was then detected in TBS (in addition to C albicans). Because the patient had developed mediastinal emphysema before, he had already been treated with amphotericin B (3 mg/kg per 24 h) to prevent mediastinitis. Upon receipt of the Candida findings, the patient first received additional caspofungin (70 mg) for one day, then micafungin (200 mg per 24 h) monotherapy from the following day on. C. auris was detected repeatedly in follow‐up blood cultures until Day 27. As a result, on Day 31 antifungal therapy was extended to include posaconazole. Subsequent blood cultures were persistently negative (Figure 1).
On Day 73, following the patient's gradual and persistent improvement, he was transferred to a rehabilitation clinic while still colonised with C. auris (TBS, skin, wound, throat and nose).
3.3. Microbiological results
Initial and follow‐up isolates of the index patient (NRZ2021‐103, OK310884 JMRC:NRZ:3148) and the contact patient (NRZ2021‐170, OK310890 JMRC:NRZ:3192) were analysed at the NRZMyk. Sequencing of the ITS region identified all isolates as C. auris (GenBank accession numbers: OK310884/OK310890). In addition, analysis of ITS revealed a possible clade affiliation to either Clade I or III, which was narrowed down to Clade I by evaluating MAT locus (Mating Type locus) sequences for all isolates. Furthermore, the very same strains harboured a clade I typical Y132F mutation in Erg11 supporting the same clade and subclone ID, thus confirming that all strains belong to C. auris clade I. 4 Antifungal susceptibility testing of the initial isolates of both patients showed nearly identical results (Table 1), and no acquisition of resistance was observed for follow‐up isolates. Sequencing the hot spots of the FKS‐gene ruled out genotypic echinocandin resistance in the presence of highly diverse echinocandin MIC values. With all C. auris isolates belonging to clade I, showing identical susceptibility patterns and marker gene sequences, no prior cases of C. auris in the unit (and the hospital) and absence of relevant risk factors in the contact patient, occurrence of the secondary case was highly likely due to in‐hospital transmission despite the very strict infection prevention measures in the index patient.
TABLE 1.
Antifungal susceptibility testing results (EUCAST); antifungal agents: anidulafungin (AND), amphotericin B (AMB), micafungin (MIC), caspofungin (CAS), 5‐flucytosine (5‐FC), posaconazole (POS), voriconazole (VOR), itraconazole (ITR) and fluconazole (FLU)
| Index patient | Contact Patient | ||
|---|---|---|---|
| NRZ‐ID‐No. | 2021‐103 (initial) | 2021‐170 (initial) | |
| Specimen | BAL | BC | |
| Minimal inhibitory concentrations (MICs) of antifungal agent (gm/L) | AND | 0.5 | 0.5 |
| AMB | 1 | 1 | |
| MIF | 0.25 | 0.125 | |
| CAS | 8 | 8 | |
| 5‐FC | 8 | 8 | |
| POS | 0.03 | 0.06 | |
| VOR | 1 | 1 | |
| ITR | 0.25 | 0.25 | |
| FLU | ≥128 | ≥128 |
3.4. Infection prevention and control measures after detection of a C. auris transmission event
Immediately after identification of the second C. auris case, an ad hoc, multi‐disciplinary outbreak panel was established in view of the high potential of this organism to cause wide‐spread outbreaks. The panel included members from the following institutions/areas: Institute of Hygiene and Environmental Medicine (IPC Department), Department of Nephrology and Medical Intensive Care, in‐house laboratory, National Reference Center for Invasive Fungal Infections, hospital's cleaning service and clinic management. The outbreak panel consented all measures described below.
The presence of IPC professionals on the ward was enhanced, and admissions to the A‐wing of the COVID‐19 ICU were stopped. Both C. auris cases were isolated in neighbouring rooms in the rear area of the ward. This area was separated from other parts of the wing by non‐occupied rooms and by means of a temporary partition wall. Separate medical equipment used across patients, such as mobile X‐ray machine, ultrasound machine, dialysis machines and ECG recorder, was set aside for these two patients only. One nurse was allocated to the two C. auris patients. We extended ongoing disinfection with a PAA disinfectant of the entire A‐wing of the ICU. Additional cleaning staff was permanently available for the intensified cleaning process and performed disinfection of all vacant patient rooms. The use of disposable blades instead of reusable ones was made mandatory for intubation controlled by video laryngoscopy. In addition, a workflow for ordering screening tests for C. auris was initiated and a ‘hygiene trigger’ in the laboratory together with an automatic electronic alert system ensured the rapid communication of findings.
3.5. Repeated cross‐sectional examinations: Patient & environmental screening
The outbreak panel initiated a comprehensive screening for C. auris on the ward. From 27 patients of the entire ICU (wings A and B) who were not affected, two swab series were obtained at a four‐day interval initially. For one series, swabs were taken from axilla, groin, wound (if applicable), throat and nose or TBS (if applicable), and a urine sample (in catheterised patients). All swabs were obtained with eSwabsTM and sent directly to the laboratory. 19 During the stay of the two C. auris patients, we conducted repeated cross‐sectional examinations of the non‐affected patients. For that, they were swabbed once or twice a week. Following negative test results, we limited this screening to the 7 patients in the A‐wing after three weeks. These patients continued to be screened for C. auris once a week until three weeks after the two C. auris patients had been discharged. None of them acquired C. auris.
Patients who had been cared for in the A‐wing of the ward were tracked back to the day the index patient was admitted, identifying a total of 20 patients. Five patients had been transferred within the hospital. For those, two swab series four days apart were analysed with no detection of C. auris. Four patients had been transferred externally. At least one swab series was analysed for these patients, with all result negative for C. auris. Out of 11 patients who had been discharged home, four had another contact to the healthcare system and were tested with one swab series. Again, no C. auris cases were identified among these patients.
Environmental swabs aimed to identify possible sources of C. auris outside the close surrounding of the C. auris patients. These swabs were taken from fixed surfaces, medical devices, and mobile equipment, including supply carts used for medical interventions and care of various patients. In addition, patient rooms were examined to determine whether C. auris could be detected there after adequate cleaning and disinfection using PAA‐based products. Of 119 environmental swabs, none tested positive for C. auris.
3.6. Identification of route of transmission
Direct transmission by staff seemed unlikely due to the 1:1 care that had been provided to the index case since admission and the fact that there was high compliance among all healthcare workers with personal protective equipment (PPE) as recommended for the care of COVID‐19 patients. Gowns and gloves were regularly changed between caring for two patients. Hand disinfection had been firmly established in compliance with the Five Moments of WHO. 20 Therefore, our analysis focused on indirect transmission via medical devices or equipment. We detected an association between the two C. auris patients for the reusable blades used in the video laryngoscope‐guided intubation. These blades were used on the index patient and seven days later in the contact case, and no other patient was intubated in the meantime. Because of the temporal relationship and the direct contact with colonised mucous membranes, we considered the reusable blades as the most probable vehicle of transmission of C. auris. These devices are routinely disinfected by the physician who used them with a chlorine dioxide‐based product that is highly effective against C. auris. 15 However, due to the difficult design of the edges and channels of the blades, the reprocessing is time‐consuming and complex (Figure 2). The blades used for video laryngoscope‐guided intubation on the ward as well as the other equipment for video laryngoscopy were examined for the presence of C. auris. However, despite a likely link to the transmission event, C. auris was not detected.
FIGURE 2.

Reusable blade used for the video laryngoscope‐guided intubation in the COVID‐19 intensive care unit and identified as the likely route of C. auris transmission
3.7. Follow‐up of the patients
The index patient visited her general practitioner on Day 57 after discharge. One swab series was negative for C. auris (throat swab positive for C albicans). On Day 105 after discharge, the index patient was again re‐admitted to our hospital due to an adrenal insufficiency; swab series and urine sample were without evidence of C. auris.
The contact patient was re‐admitted to an external hospital because of his chronic lung disease 45 days after discharge. One swab series still revealed evidence of C. auris colonisation (throat swab). Additional control screenings were conducted at the Infectious Diseases Outpatient Clinic of our hospital (Days 118, 139 and 160 after discharge). These three swab series and one sputum sample were negative for C. auris. On Day 202 after discharge, the contact patient was again re‐admitted to our hospital for operative closure of the tracheostoma. At this time, while the swab series were negative, C. auris was detected in a sputum sample, indicating long‐term colonisation.
4. DISCUSSION
We report the patient‐to‐patient transmission of C. auris in a COVID‐19 ICU and the successful measures taken to prevent a hospital outbreak. Transmission occurred despite the fact that the index patient had been strictly isolated since admission due to CRKP colonisation. Likely this is one reason why C. auris did not spread extensively on the ward as described in other outbreaks. 1 , 21 , 22 Due to the strict isolation measures, the possibility of transmission via medical devices or other equipment used across several patients was most likely, in line with known routes for C. auris transmission. 21 , 23 We determined that the same set of laryngoscope blades had been used sequentially in both patients. C. auris was detectable in the TBS of both patients, which is consistent with transmission via the pharyngo‐tracheal route. Manual reprocessing of the blades with chlorine dioxide by the physician who used them for intubation of the index patient may have been insufficient. Although this mode of reprocessing is possible according to the manufacturer's instructions, machine reprocessing of medical devices is generally preferable to manual reprocessing because of its higher reliability, traceability and opportunities for validation. 24 Alternatively, disposable blades can be used. These have since then been introduced in our ICU.
After identification of C. auris transmission, an entire bundle of IPC measures was promptly implemented on the ward and in the diagnostic facilities used for patient care. 25 Given our lack of experience in controlling C. auris, uncertainty arose among the staff. In a Cochrane review on interventions to promote resilience and mental health among healthcare workers in the context of outbreaks, the authors identified two factors that had a positive impact: effective communication and a supportive learning environment. 26 Accordingly, we educated the medical and other associated staff about the epidemiology, biology and prevention of transmission of the fungus. IPC specialists supported the implementation of IPC measures on a daily basis on the ward. Once these measures had been properly implemented, no further patients showed evidence of C. auris. Importantly the initiated measures prevented further spread of C. auris. Interestingly, and in contrast to other outbreak reports, we did not observe environmental contamination with C. auris. The reason remains unclear but could be related to isolation of the index patient and the strict environmental disinfection protocols in place at a COVID‐19 unit.
Due to the severity of disease and the need for ICU treatment of COVID‐19, these patients are particularly susceptible to fungal infections, including C. auris. 10 , 23 , 27 , 28 , 29 This requires an increased awareness of the risk of C. auris in patients coming from regions with an endemic occurrence. In the United States, all patients who have stayed overnight in a healthcare facility outside the United States within the past year are supposed to be screened for C. auris. 30 Up to now, such routine admission screening for patients at high risk of C. auris have not been recommended in Germany, where C. auris is still very rare. Intrinsic multidrug resistance complicates the treatment of C. auris. 3 , 31 Here, follow‐up blood cultures from the patient who developed a C. auris bloodstream infection remained positive despite combined liposomal amphotericin B and echinocandin treatment and were only cleared after initiation of posaconazole.
Finally, our observations in the contact patient confirm that colonisation with C. auris can persist over prolonged time periods and can re‐occur even after negative test series. Thus, patients once colonised with C. auris should be treated with appropriate infection prevention measures when re‐admitted to a healthcare setting. Recent data show that while intestinal C. auris colonisation is less frequent than skin colonisation, it may be more stably detectable. In addition, intestinal carriage pre‐disposes urinary tract infections caused by C. auris. 32 Based on our experience, we have set up recommendations for appropriate handling of C. auris in Germany (Aldejohann AM, Wiese‐Posselt M, Gastmeier P, Kurzai O. Expert recommendations for prevention and management of Candida auris transmission. Mycoses 2022 Apr 19. doi: 10.1111/myc.13445. Online ahead of print. PMID: 35437832).
CONFLICT OF INTEREST
CH, MWP, BG, CG, BW, PE, AA, AS, AK, KUE, PG and OK do not report conflict of interest.
AUTHOR CONTRIBUTIONS
CH, MWP, CG, BW, PE, AS, AK, KUE and PG were part of an interdisciplinary team responsible for the care of the two reported patients and for measures to track and control the infection transmission. OK provided important expert advice during for transmission control and patient management. BG, AS, AK, AA and OK conducted and interpreted the laboratory work. CH and MWP wrote the first draft of the manuscript. All other authors actively reviewed the manuscript and approved the final version.
ACKNOWLEDGEMENTS
We would like to thank all healthcare personnel of Charité Universitätsmedizin Berlin and the staff of Charité CFM Facility Management, who were involved in the care and treatment of the patients. We would especially like to thank the COVID‐19 ICU ward managers Anett Hoerenz, Sebastian Rieks, and Enrico Schlunk, as well as Kathrin Labahn and Hendrikje Hensel from the Institute of Hygiene and Environmental Medicine. We are grateful to Mast Diagnostica GmbH (Reinfeld, Germany) for providing CHROMagar Candida PlusTM agar plates. Moreover, we thank Gerald Bennan for the proofreading of the manuscript and the anonymous reviewers for multiple helpful comments.
Hinrichs C, Wiese‐Posselt M, Graf B, et al. Successful control of Candida auris transmission in a German COVID‐19 intensive care unit. Mycoses. 2022;65:643–649. doi: 10.1111/myc.13443
Funding information
Work in the NRZMyk is supported by the Robert Koch Institute with funds provided by the German Ministry of Health (grant 1369‐240)
Carl Hinrichs and Miriam Wiese‐Posselt should be considered joint first author.
Contributor Information
Carl Hinrichs, Email: carl.hinrichs@charite.de.
Miriam Wiese‐Posselt, Email: miriam.wiese-posselt@charite.de.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Schelenz S, Hagen F, Rhodes JL, et al. First hospital outbreak of the globally emerging candida auris in a European hospital. Antimicrob Resist Infect Control. 2016;5:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bidaud AL, Chowdhary A, Dannaoui E. Candida auris: an emerging drug resistant yeast ‐ a mini‐review. J Mycol Med. 2018;28(3):568‐573. [DOI] [PubMed] [Google Scholar]
- 3. Du H, Bing J, Hu T, Ennis CL, Nobile CJ, Huang G. Candida auris: Epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog. 2020;16(10):e1008921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chow NA, Munoz JF, Gade L, et al. Tracing the evolutionary history and global expansion of candida auris using population genomic analyses. MBio. 2020;11(2):e03364‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. von Lilienfeld‐Toal M, Wagener J, Einsele H, Cornely OA, Kurzai O. Invasive fungal infection. Dtsch Arztebl Int. 2019;116(16):271‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Schalkwyk E, Mpembe RS, Thomas J, et al. Epidemiologic shift in candidemia driven by candida auris, South Africa, 2016–2017(1). Emerg Infect Dis. 2019;25(9):1698‐1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kohlenberg A, Struelens MJ, Monnet DL, Plachouras D. The candida auris survey collaborative G. candida auris: epidemiological situation, laboratory capacity and preparedness in European Union and European Economic Area countries, 2013 to 2017. Euro Surveill. 2018;23(13):18‐00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Plachouras D, Lotsch F, Kohlenberg A, Monnet DL, Candida auris: epidemiological situation, laboratory capacity and preparedness in the European Union and European Economic Area*, January 2018 to May 2019. Eurosurveillance. 2020;25(12):2000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hamprecht A, Barber AE, Mellinghoff SC, et al. Candida auris in Germany and previous exposure to foreign healthcare. Emerg Infect Dis. 2019;25(9):1763‐1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prestel C, Anderson E, Forsberg K, et al. Candida auris outbreak in a COVID‐19 specialty care unit ‐ florida, july‐august 2020. MMWR Morb Mortal Wkly Rep. 2021;70(2):56‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Usyk M, Zolnik CP, Patel H, Levi MH, Burk RD. Novel ITS1 fungal primers for characterization of the mycobiome. mSphere. 2017;2(6):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aldejohann AM, Herz M, Martin R, Walther G, Kurzai O. Emergence of resistant candida glabrata in Germany. JAC Antimicrob Resist. 2021;3(3):dlab122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arendrup MC, Meletiadis J, Mouton JW, Lagrou K, Hamal P. Guinea J and the Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). EUCAST DEFINITIVE DOCUMENT E.DEF 7.3.2. Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts. 2020. https://wwweucastorg/fileadmin/src/media/PDFs/EUCAST_files/AFST/Files/EUCAST_E_Def_732_Yeast_testing_definitive_revised_2020pdf. Last Accessed on 2021‐10‐29.
- 14. Gastmeier P, Kampf KP, Behnke M, Geffers C, Schwab F. An observational study of the universal use of octenidine to decrease nosocomial bloodstream infections and MDR organisms. J Antimicrob Chemother. 2016;71(9):2569‐2576. [DOI] [PubMed] [Google Scholar]
- 15. Abdolrasouli A, Armstrong‐James D, Ryan L, Schelenz S. In vitro efficacy of disinfectants utilised for skin decolonisation and environmental decontamination during a hospital outbreak with Candida auris. Mycoses. 2017;60(11):758‐763. [DOI] [PubMed] [Google Scholar]
- 16. Muller P, Tan CK, Issleib U, Passvogel L, Eilts B, Steinhauer K. Investigation of the susceptibility of Candida auris and Candida albicans to chemical disinfectants using European Standards EN 13624 and EN 16615. J Hosp Infect. 2020;105(4):648‐656. [DOI] [PubMed] [Google Scholar]
- 17. Rutala WA, Kanamori H, Gergen MF, Sickbert‐Bennett EE, Weber DJ. Susceptibility of Candida auris and Candida albicans to 21 germicides used in healthcare facilities. Infect Control Hosp Epidemiol. 2019;40(3):380‐382. [DOI] [PubMed] [Google Scholar]
- 18. Cadnum JL, Shaikh AA, Piedrahita CT, et al. Effectiveness of disinfectants against candida auris and other candida species. Infect Control Hosp Epidemiol. 2017;38(10):1240‐1243. [DOI] [PubMed] [Google Scholar]
- 19. Copan Italia s.p.a.: eSwab™. 2021. https://mediadeliverycopangroupcom/wp‐content/uploads/2021/10/eswab_JMKPF001R00_DIGITALpdf. Last Access: 2021‐12‐07.
- 20. World Health Organization (WHO) . Your 5 moments for hand hygiene. 2009. https://wwwwhoint/gpsc/5may/Your_5_Moments_For_Hand_Hygiene_Posterpdf. Last Access 2021‐09‐14.
- 21. Eyre DW, Sheppard AE, Madder H, et al. A candida auris outbreak and its control in an intensive care setting. N Engl J Med. 2018;379(14):1322‐1331. [DOI] [PubMed] [Google Scholar]
- 22. Garcia CS, Palop NT, Bayona JVM, et al. Candida auris: report of an outbreak. Enferm Infecc Microbiol Clin (Engl Ed). 2020;38(Suppl. 1):39‐44. [DOI] [PubMed] [Google Scholar]
- 23. Nobrega de Almeida J, Jr , Brandao IB, Francisco EC, et al. Axillary digital thermometers uplifted a multidrug‐susceptible candida auris outbreak among COVID‐19 patients in Brazil. Mycoses. 2021;64(9):1062‐1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koch‐Institute R. Empfehlungen der Kommission für Krankenhaushygiene und Infektionsprävention: hygiene requirements for the reprocesssing ofo medical devices. Bundesgesundheitsblatt ‐ Gesundheitsforschung ‐ Gesundheitsschutz. 2012;10:1244‐1310. [DOI] [PubMed] [Google Scholar]
- 25. European Centre for Disease Prevention and Control, Stockholm . Candida auris in healthcare settings – Europe, first update, 23 April 2018. 2018. https://wwwecdceuropaeu/en/publications‐data/rapid‐risk‐assessment‐candida‐auris‐healthcare‐settings‐europe. Last Accessed on 2021‐10‐29.
- 26. Pollock A, Campbell P, Cheyne J, et al. Interventions to support the resilience and mental health of frontline health and social care professionals during and after a disease outbreak, epidemic or pandemic: a mixed methods systematic review. Cochrane Database Syst Rev. 2020;2020(11):CD013779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chowdhary A, Tarai B, Singh A, Sharma A. Multidrug‐resistant candida auris infections in critically Ill coronavirus disease patients, India, April‐July 2020. Emerg Infect Dis. 2020;26(11):2694‐2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thoma R, Seneghini M, Seiffert SN, et al. The challenge of preventing and containing outbreaks of multidrug‐resistant organisms and Candida auris during the coronavirus disease 2019 pandemic: report of a carbapenem‐resistant Acinetobacter baumannii outbreak and a systematic review of the literature. Antimicrob Resist Infect Control. 2022;11(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Seagle EE, Jackson BR, Lockhart SR, et al. The landscape of candidemia during the COVID‐19 pandemic. Clin Infect Dis. 2021;74(5):802‐811. doi: 10.1093/cid/ciab562 [DOI] [PubMed] [Google Scholar]
- 30. Centers for Disease Control and Prevention (CDC) > Fungal Diseases > Candida auris. https://wwwcdcgov/fungal/candida‐auris/indexhtml. Last accessed on October 5, 2021.
- 31. Arensman K, Miller JL, Chiang A, et al. Clinical outcomes of patients treated for candida auris infections in a multisite health system, Illinois, USA. Emerg Infect Dis. 2020;26(5):876‐880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Piatti G, Sartini M, Cusato C, Schito AM. Colonization by candida auris in critically ill patients: role of cutaneous and rectal localization during an outbreak. J Hosp Infect. 2022;120:85‐89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


