FIGURE 3.
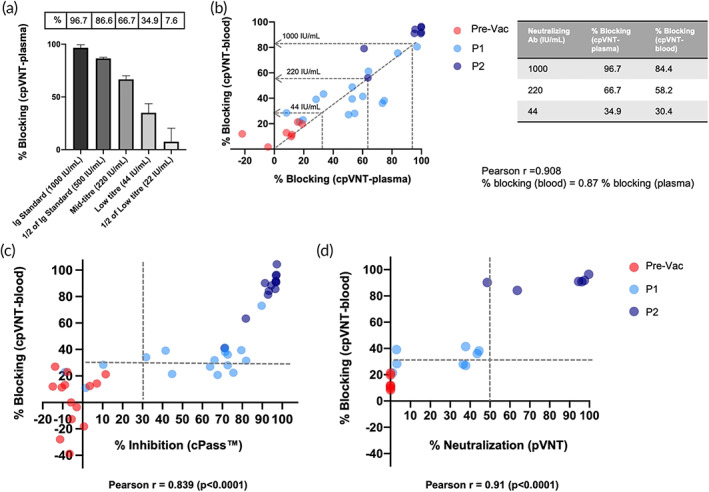
Comparison of modified cpVNT with international standards and established serology tests. (a) The performance of the First WHO International Standard Anti‐SARS‐CoV‐2 immunoglobulin (20/136), Reference Panel for anti‐SARS‐CoV‐2 Mid‐tire and Low‐titre plasma using modified cpVNT. (b) The correlation of percent blocking measured from 30 matching plasma and blood samples at prevaccination (Pre‐Vac), post first dose (P1) and post second dose (P2) phase using modified cpVNT gave Pearson r, 0.908. The percent blocking for cpVNT with blood samples that correspond to 1000, 220, and 44 IU/ml are determined by assuming a linear correlation between the two sample types (see accompanying table). (c) Comparison of percent blocking measured in the modified cpVNT with percent inhibition of sVNT (cPass) in 44 matching Pre‐Vac, P1, and P2 venous blood and plasma samples. The sensitivity was calculated as 81.5% (CI: 61.9%–93.7%), and specificity is 100% (CI: 81.5%–100%) when both cpVNT and sVNT's (cPass) thresholds were set at 30% blocking. (d) Comparison between cpVNT and pseudovirus neutralization test (pVNT) with 20 individuals' sample. The pVNT was performed with plasma in 1:80 dilution. The sensitivity is 100% (CI: 47.8%–99.9%) and specificity is 66.7% (CI: 38.4%–88.2%) with 30% blocking as the threshold for cpVNT and 50% neutralization for pVNT. All experiments were performed in triplicates. CI, confidence interval; cpVNT, cellulose pulldown virus neutralization test; nAb, neutralizing antibody; pVNT, pseudovirus neutralization test
