Abstract
Over the course of the COVID-19 pandemic, wastewater surveillance has become a useful tool for describing SARS-CoV-2 prevalence in populations of varying size, from individual facilities (e.g., university residence halls, nursing homes, prisons) to entire municipalities. Wastewater analysis for SARS-CoV-2 RNA requires specialized equipment, expensive consumables, and expert staff, limiting its feasibility and scalability. Further, the extremely labile nature of viral RNA complicates sample transportation, especially in regions with limited access to reliable cold chains. Here, we present a new method for wastewater analysis, termed exclusion-based sample preparation (ESP), that substantially simplifies workflow (at least 70% decrease in time, 40% decrease in consumable usage compared to traditional techniques) by targeting the labor-intensive processing steps of RNA purification and concentration. To optimize and validate this method, we analyzed wastewater samples from residence halls at the University of Kentucky, of which 34% (44/129) contained detectible SARS-CoV-2 RNA. Although concurrent clinical testing was not comprehensive, student infections were identified in the seven days following a positive wastewater detection in 68% of samples. This pilot study among university residence halls validated the performance and utility of the ESP method, laying the foundation for future studies in regions of the world where wastewater testing is not currently feasible.
Keywords: COVID-19, wastewater, assays, nucleic acids, epidemiology
Graphical Abstract
Synopsis:
This work expands on the design process of an efficient and simplified wastewater-based pathogen surveillance methodology and its application in public health.
Introduction
Wastewater-based surveillance of SARS-CoV-2 emerged recently as a strategy for monitoring disease prevalence, as populations can be surveilled by testing a single wastewater sample for the presence of viral biomarkers1. As SARS-CoV-2 is shed in the stool of infected people2, wastewater surveillance overcomes several limitations of clinical surveillance, such as the need for high throughput laboratory infrastructure3 and the logistical challenges associated with sampling everyone within a population. Correlations between wastewater SARS-CoV-2 RNA levels and clinical cases at both community4,5 and facility6 levels have been reported. Additionally, rising SARS-CoV-2 concentration levels detected in wastewater samples may predate rising case numbers of COVID-197,8 and may identify isolated infections9. Wastewater surveillance for SARS-CoV-2 is rapidly growing in the United States, and the Centers for Disease Control and Prevention (CDC) initiated a National Wastewater Surveillance System (NWSS) to pool data from voluntary partners across the country, although this effort is currently focused primarily on samples collected from wastewater treatment plants10. Consequently, several research groups – including our own – have begun collecting effluent wastewater from congregate living facilities6,11,12 (e.g., residence halls, nursing homes) to identify COVID-19 “hotspots” and facilitate targeted clinical testing.
Although wastewater surveillance provides valuable information about the spread of COVID-19, several challenges limit widespread implementation, particularly in rural/remote regions and low- and middle-income countries (LMICs). First, SARS-CoV-2 RNA concentrations in wastewater (typically ranging from 10s to 1,000s of copies per mL) are much lower than in clinical samples due to dilution with non-fecal wastewater (e.g., drainage from laundry, showers, or kitchen activities). Second, within a wastewater matrix, SARS-CoV-2 RNA degrades in mere hours and cold chain transportation does not guarantee long term stability13. Third, traditional wastewater analysis protocols often utilize multiple complex filtration or centrifugation steps, requiring specialized equipment. These limitations have largely restricted SARS-CoV-2 wastewater testing to locations adjacent to sophisticated molecular biology laboratories (e.g., congregate living settings near major research institutions) or, at a minimum, regions with reliable rapid shipping to such facilities. To overcome these restrictions, RNA extraction methods must be highly efficient and/or provide significant concentration of SARS-CoV-2 RNA. Similarly, analysis (or RNA stabilization) must be performed quickly, with minimal shipping and workflow delays. If we are to overcome the technical and logistical challenges associated with SARS-CoV-2 wastewater testing, we must simplify the methodologies used in wastewater analysis. To this end, we focus here on the most labor-intensive components of analysis: RNA extraction and concentration. Specifically, we applied a new RNA extraction and concentration technology, ESP, to wastewater analysis.
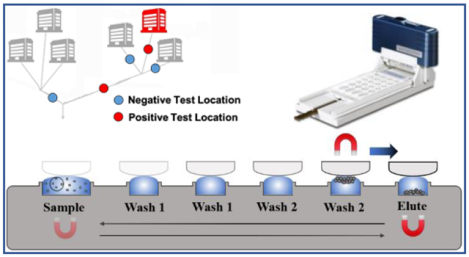
ESP provides a fast, simple, and electricity-free means of manipulating RNA. Prior to this study, ESP has been used in other applications14–19. These include the successful quantitative analysis of HIV viral load from clinical samples in Uganda,14 demonstrating that although non-extracted viral HIV RNA is extremely sensitive to degradation, ESP-extracted HIV RNA is relatively stable at ambient temperature for three days. ESP simplifies existing processing technologies (Figure 1) by drastically reducing both extraction time and the usage of consumable supplies – metrics which are highly relevant in remote and/or resource-limited areas lacking expansive laboratory space and/or highly skilled personnel.
Figure 1:
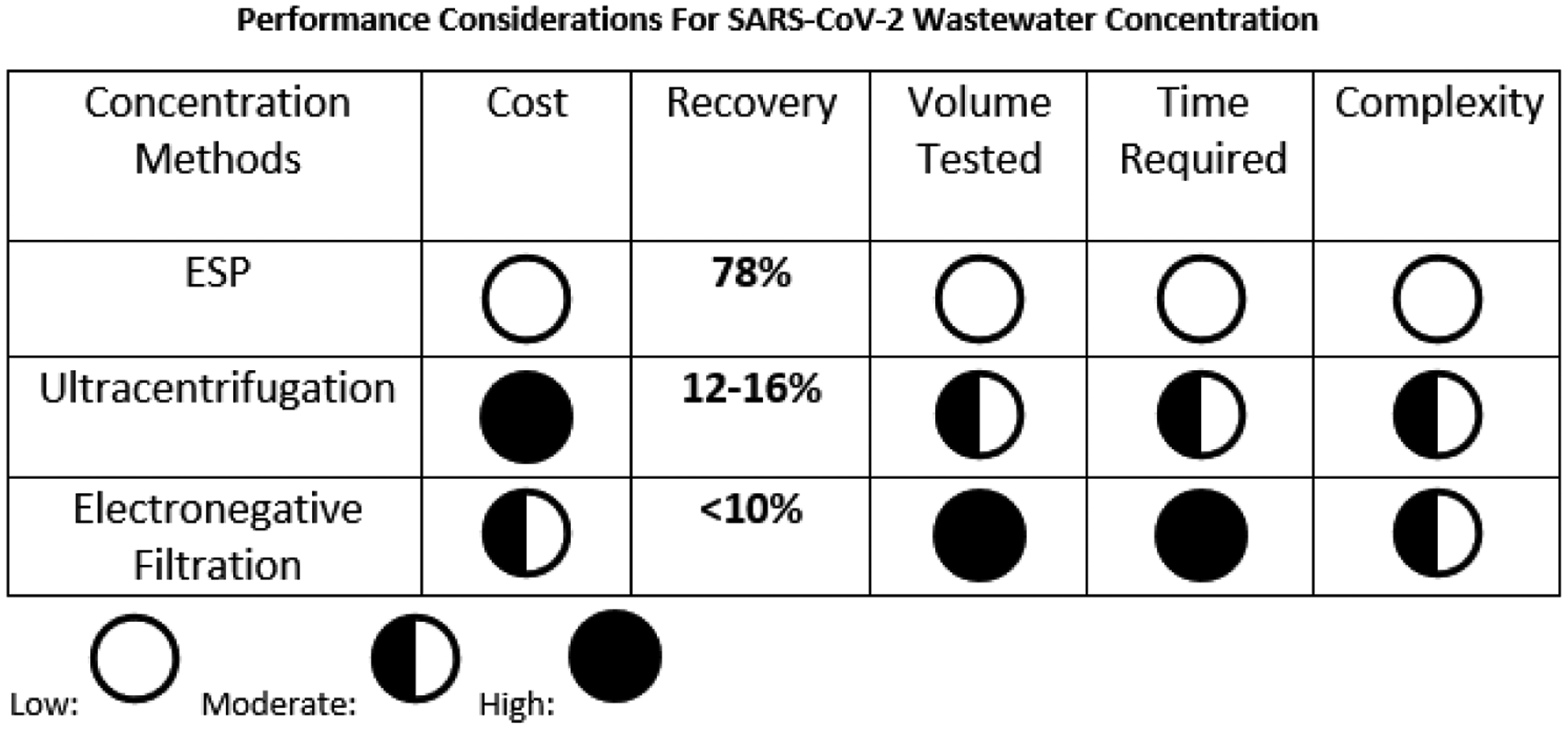
A comparison of ESP with existing wastewater analysis processes11,36,37. Additional details on the comparator methods are given in a review by Rusiñol et al.38.
This manuscript represents the first application of ESP to wastewater processing. We developed an analytical process that sensitively detected SARS-CoV-2 without the use of costly laboratory equipment (e.g., ultracentrifuges) or expensive single-use consumables (e.g., ultrafiltration). This new application of ESP technology included optimization for wastewater samples, benchmarking of performance, and validation using wastewater samples from university dormitories during an academic year.
Materials and Methods
Collection of Samples
While our ESP-based method is particularly well-suited to address the challenges associated with wastewater analysis in remote settings (e.g., LMIC countries), our ability to collect wastewater from such locations was limited during the pandemic. Therefore, we evaluated the performance of the ESP-based protocol using wastewater samples collected from residence halls on the University of Kentucky campus. We used an autosampler (ISCO GLS) to collect composite wastewater effluent samples (100 mL fractions every 20 minutes during a 4–24 hour sampling window). Low-flow strainers were used at locations with sewer architecture that limited flow depth. An emphasis on sampling the early morning hours (7:00–9:00am) was chosen to reflect the morning bathroom routines of students contributes. Transit time from the toilet to the collection site was explored by flushing a food-grade dye and unique RNA sequence into the waste system at a similarly sized residential building and collecting samples in 5-minute intervals using an ISCO 3700c composite sampler (see Supplementary Information for additional details). From these experiments, we determined that the 20-minute sampling window is sufficient to capture a flushed biomarker (see Supplementary Information). A bottle containing 100–200 mL of collected wastewater from the autosampler was transferred on ice to the laboratory for analysis. During the Fall 2020 semester, sample locations were selected by the University of Kentucky Emergency Operations Center based on sewer infrastructure and trends in COVID-19 infections. During the Spring 2021 semester, weekly composite samples were taken from five campus sewersheds servicing two to five residence halls. When a high (typically >200 cp/mL) SARS-CoV-2 wastewater signal was observed, effluent from the associated building was sampled later in the week (Figure 2). Therefore, we were able to streamline wastewater surveillance from multi-facility sub-groups to individual buildings by reacting to the most recent data. Wastewater samples were obtained for all academic weeks except for the week of February 16, 2021 due to severe weather.
Figure 2:
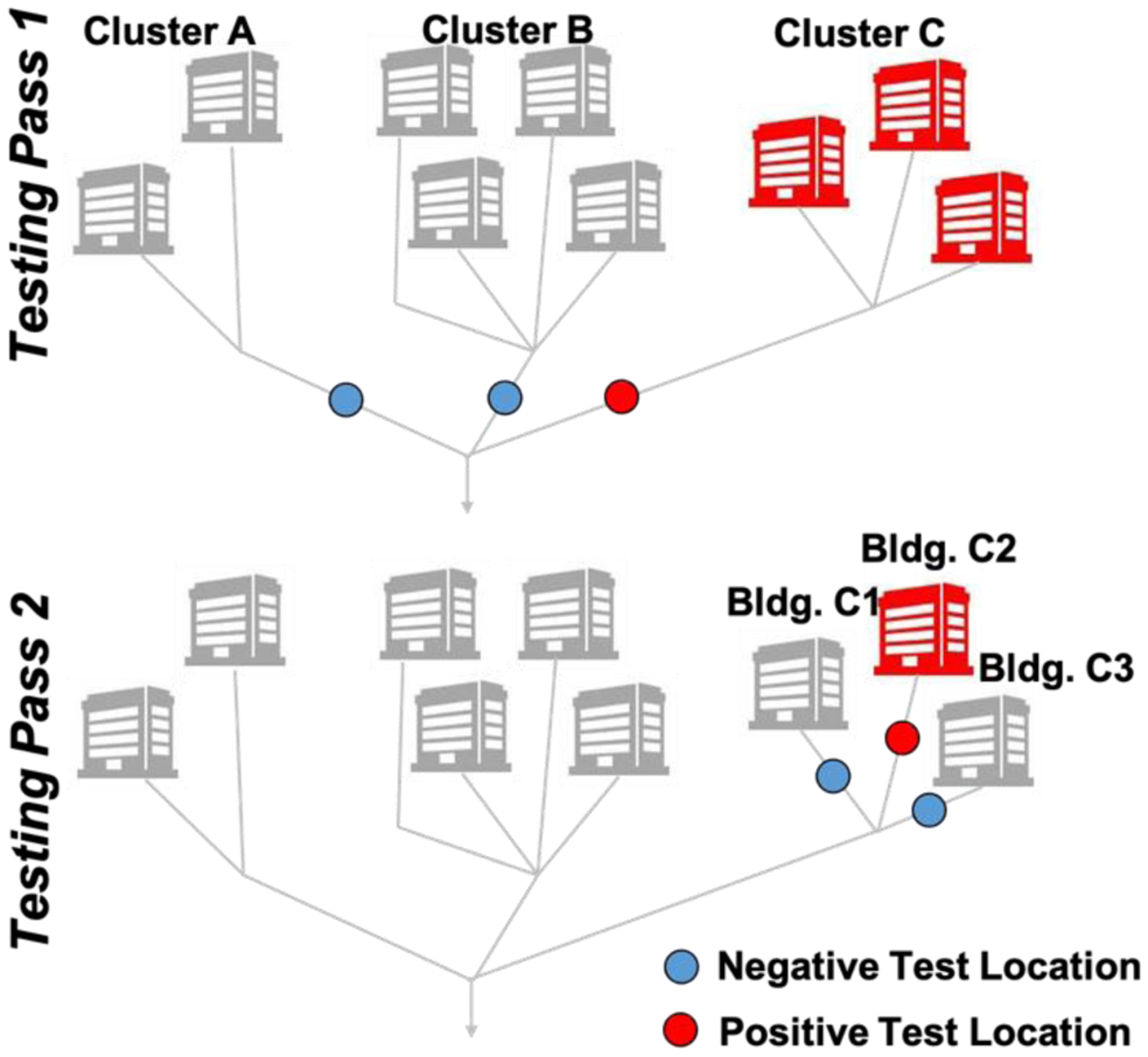
Our residence hall sampling strategy involved two testing passes per week. In the first pass, clusters of buildings were sampled at downstream access points. A second testing pass refined the location of hotspots by sampling at access points representing single buildings.
RNA Extraction Process
Lysis and PMP Binding:
RNA was extracted from wastewater samples on the same day as sample collection. Because wastewater is heterogenous, the sample bottle was inverted several times and mixed on a stir plate prior to removing aliquots for analysis. For each sample, we extracted RNA from eight 250 μL replicates of wastewater using ESP, allowing for characterization of sample heterogeneity. Viral particles were lysed in a buffer containing 4 M guanidine thiocyanate (ThermoFisher) and 10 mM 4-morpholineethanesulfonic acid (MES) sodium salt (Sigma Aldrich) dissolved in absolute ethanol with 1.5 times of total sample volume. Lysis buffer and wastewater samples were mixed in a 3:2 ratio in order to maintain a high-salt environment conducive to RNA capture. Paramagnetic particles (PMPs) were subsequently added to each microcentrifuge tube. Prior studies involving ESP demonstrated that PMP type selection correlated significantly with assay outcome20. Therefore, we compared four different PMP types, comparing capture percentage using spiked wastewater samples. PMPs included MagAttract Suspension G (Qiagen, # 1026883), MagneSil Paramagnetic Particles (Promega, #MD1360), Abbott mDNA Microparticles (Promega) and SeraSil-Mag (Cytiva, #29357369 and #29357374). Eight replicates were performed for each comparison and RT-qPCR was utilized to compare measured RNA concentration with spiked RNA concentration. Comparison was performed using a paired Student’s t-test on each combination of factors followed by a Bonferroni correction for this family of comparisons performed with an α = 0.05. Following results of this optimization experiment, 20 μL of the selected PMPs (Cytiva, see Results and Interpretation) were pipetted into each microcentrifuge tube. Each sample was then vortexed for 15–20 seconds and heated at 50°C for 20 minutes with intermittent mixing to accelerate viral lysis. While heat-inactivation has been shown to under similar conditions21, recent data suggests that there is no infectious SARS-CoV-2 present in wastewater22. Afterwards, tubes were tumbled for 20 minutes and centrifuged at low speed to collect PMPs or liquid that had adhered to the sides or cap of the tube. The supernatant was removed from each sample by holding a magnet to the side of the tube for approximately 30 seconds to immobilize PMPs. After the liquid was removed, 500 μL of Wash Buffer 1 was added to the PMPs and mixed via pipette. Wash Buffer 1 was made by mixing 1M guanidine thiocyanate (ThermoFisher), 10 mM Tris buffer pH 8 (ThermoFisher), and 1% Tween® 20 solution (Sigma-Aldrich) with 990 ml of distilled water. Wash buffer 2 was made by mixing 1M guanidine thiocyanate (ThermoFisher) and 10 mM Tris buffer pH 8 (ThermoFisher) with 990 ml of absolute alcohol. Buffer recipes were modified from those previously described by Escobar et al.23
ESP RNA Purification:
To rapidly and efficiently purify and concentrate the PMP-captured viral RNA, a commercially available ESP device known as the Extractman (Gilson, Inc.) was used. The Extractman consists of a magnetic slider that moves over a magnetic base. The motion of this slider transfers PMPs through several air/liquid phase interfaces, thereby efficiently excluding PMP-bound RNA from unbound material that would otherwise inhibit downstream RT-qPCR. The materials and geometry of the Extractman consumables were selected to minimize any unbound liquid carryover that may have occurred during the purification process. Specifically, this involved selecting a hydrophobic material (polypropylene, in our case) and a consumable geometry that minimized plastic/liquid contact as well as unnecessary wetting of solid surfaces. Prior to operation, a polypropylene Extractman plate (Gilson, Inc.) was loaded with samples and wash buffers. 500 μL of sample/PMP mixture was transferred to Well 1 of the Extractman plate. The Extractman plate was loaded with Wash Buffers 1 and 2 in Wells 1 through 5 and an elution buffer of nuclease-free water in Well 6 as detailed in Figure 3. To operate the ESP device, a sterile Extractman bead capture strip (Gilson, inc.) was loaded onto the Extractman handle (Gilson, Inc.). The sliding magnet on the Extractman base was moved away from Well 1 to promote collection of the PMPs onto the extraction strip. Once all PMPs were collected on the strip (approximately 20 seconds), the Extractman slider was moved above Well 2 while the sliding base magnet was positioned beneath Well 2 to release the PMPs into the wash buffer solution. The base magnet was then moved back and forth 15 times to actuate the PMPs in an up and down motion and promote mixing. This method was repeated for the remaining wells in each column with wash buffers. For Wells 2 and 3, 15 magnetic mixes were performed. In Wells 4 and 5, only 5 washes were performed. At the completion of the washes, the PMPs were deposited into the elution buffer in Well 6, resulting in a 2.5 factor concentration due to volumetric reduction (Figure 3C). No washing was required at this step; once all beads dropped from the extraction strip, the ESP slider was removed from the base. Using a pipette, the contents of Well 6 (now containing purified PMP-RNA complexes) were collected and placed in a sterile microcentrifuge tube, which was subsequently heated for 20 minutes at 70°C to accelerate elution of the RNA from the PMPs. Following elution, PMPs were immobilized on a magnetic stand, allowing RNA to be collected and placed into a new microcentrifuge tube. RNA was either stored at −80°C or quantified immediately via RT-qPCR.
Figure 3:
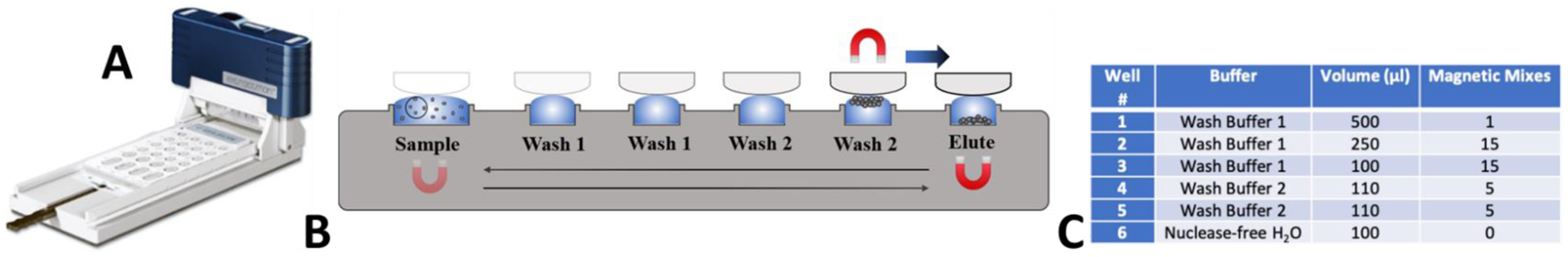
A) Photo of ESP device. B) Illustration of ESP operation, highlighting the movement of beads through a series of wells using magnets above and below the ESP well plate. C) Description of buffer and volume loaded into each of the labeled wells in Panel B.
Comparison RNA Purification:
As a comparator to ESP, we also performed extraction using a traditional PMP extraction protocol. Samples were lysed and RNA was bound to PMPs as previously described. Instead of using ESP to perform the four washes and elution, each wash buffer (using identical buffers to the ESP protocol) was added via pipette and mixed for ~15 seconds to resuspend the PMPs. Sample tubes were then placed on a magnetic rack and left for ~1 minute to facilitate collection of the PMPs. Buffer was removed from each sample tube via pipetting and the tubes were then removed from the rack. This process was repeated for each wash buffer. This process was then compared to the ESP-based protocol to determine time and operational complexity advantages.
RT-qPCR
ESP-purified RNA was amplified and quantified via RT-qPCR using the CDC-recommended24 SARS-CoV-2 N1 gene primer and probe sequences (forward primer: 5’-GACCCCAAAATCAGCGAAAT-3’, reverse primer: 5’-TCTGGTTACTGCCAGTTGAATCTG-3’, probe: 5’-ACCCCGCATTACGTTTGGTGGACC-3’ with FAM fluorophore and MGB quencher). The RT-qPCR reaction mixtures had a total volume of 20 μl and included 5 μL of TaqMan™ 4X Fast Virus 1-Step Master Mix (Applied Biosystems), 1 μL of primer and probe (custom synthesized by ThermoFisher at 60X and then diluted to a working concentration of 20X), 4 μl of nuclease-free water, and 10 μl of extracted RNA as template. Preliminary comparisons between the CDC’s N1 and N2 assays yielded stronger signal for the N1 assay, prompting us to adopt this assay as our primary readout (results not shown). All PCR assays were conducted with a LightCycler® 480 II (Roche Diagnostics). Positive and negative controls were added to each PCR plate to ensure the validity of the PCR process. For the positive control, a spike of SARS-CoV-2 RNA (isolate USA-WA1/2020, obtained from BEI Resources) was added to the reaction at a concentration of approximately 500 copies/reaction. The RT-qPCR program consisted of reverse transcription at 50°C for 5 minutes and a 20 second hot start at 95°C, followed by 45 cycles of the following protocol: 1) 95°C for 15 seconds; 2) 60°C for one minute; and 3) real-time fluorescence measurement for FAM signal. The assay limit of detection (LoD) was calculated by measuring the signal obtained from SARS-CoV-2-negative wastewater spiked with heat-inactivated SARS-CoV-2 RNA (NR-52286) at 100–10,000 copies per mL
Data Analysis
Wastewater SARS-CoV-2 concentrations were calculated based on cycle threshold (CT) values using the Roche LightCycler 2nd Derivative Maximum Algorithm. CT values were translated into SARS-CoV-2 genomic concentration using a standard curve (r2=0.985) constructed from serial dilutions of the BEI positive control RNA. Wastewater concentrations were normalized by the populations of each residence hall cluster by dividing the bulk wastewater concentration (in cp/mL) by the cluster population (A: 531, B: 1,391, C: 929) and multiplying the result by 1,000 (for visualization purposes).
Wastewater SARS-CoV-2 concentrations were scrutinized for associations with clinical data collected by the University of Kentucky. These data included the results of comprehensive student testing at the beginning of fall semester 2020 and spring semester 2021. Additional risk-based clinical testing occurred for symptomatic students (self-referred) and close contacts (University contact tracing team-referred) or following a strongly positive wastewater signal (at the discretion of the Emergency Operations Commander). Some random clinical testing of students was also conducted. Cluster wastewater data (collected Monday to Tuesday) was compared with clinical results obtained from Monday through the following Monday (i.e., students that became clinically positive within the week following each wastewater datapoint). The total number of clinical positives as well as the clinical positivity rate (i.e., positive tests / total tests) were compared to wastewater results by dormitory.
Results and Interpretation
Optimization of Protocol and Benchmarking
Based on prior studies20, we compared performance of four different PMP types on metrics of capture percentage using spiked wastewater samples. SeraSil PMPs outperformed the alternatives with an average recovery of 89% (Figure 4A), although only one of these comparisons was statistically significant (SeraSil vs. MagAttract). Additionally, our ESP-assisted method reduced washing times by roughly 70% (20–25 minutes reduced to 4–5 minutes per 8 replicates) and reduced supply use (pipette tips, tubes) by roughly 40% (34 plastic consumables reduced to 19 plastic consumables per 8 replicates) when compared to comparison RNA purification method described in the Materials and Methods section.
Figure 4:
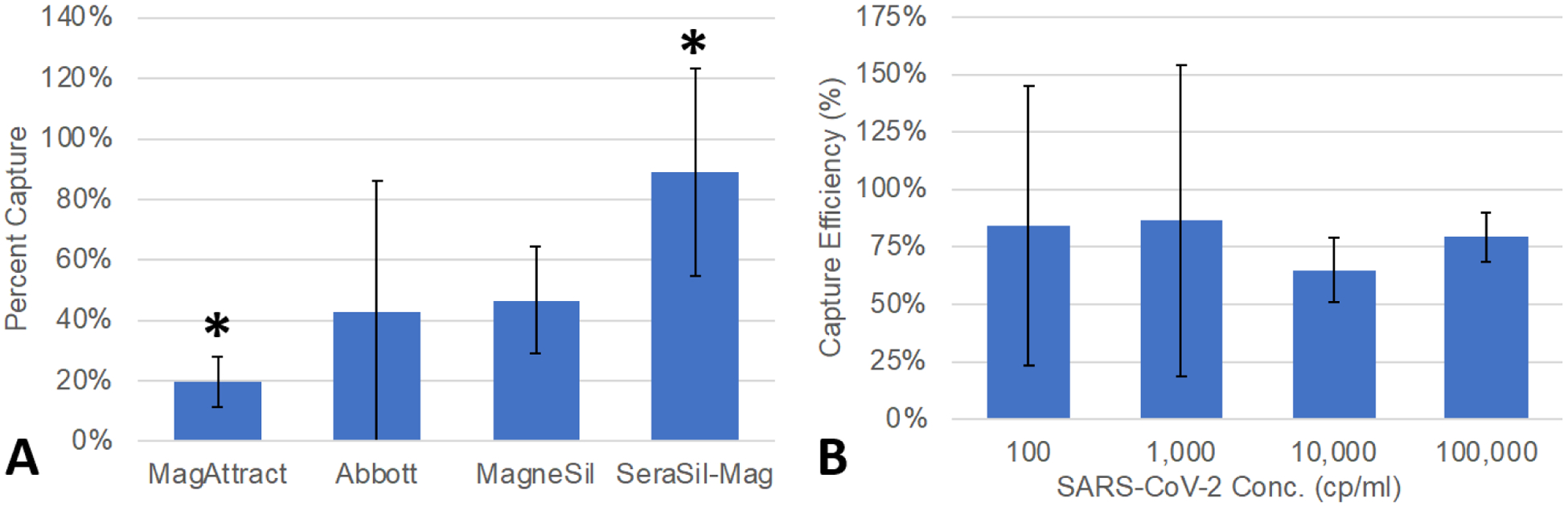
A) SeraSil captured significantly more SARS-CoV-2 RNA than other tested PMPs. *Stastistically significant as determined by 2-tailed Student’s t-test with Bonferroni correction. MagneSil and SeraSil-Mag both have lowest coefficients of variation of 38%. Error bars represent standard deviation. B) Capture efficiency of heat-inactivated SARS-CoV-2 (NR-52286) spiked wastewater. Error bars represent standard deviation.
Using SeraSil PMPs and the protocol described in the Materials and Methods section, we evaluated the LoD by spiking heat-inactivated SARS-CoV-2 RNA (NR-52286, obtained from BEI Resources) into negative wastewater (confirmed negative by an ESP-facilitated test returning eight negative aliquots). At a concentration of 1,000 copies per mL of wastewater effluent (25 copies per RT-qPCR reaction given reagent dilution), we successfully detected SARS-CoV-2 in seven out of eight replicates (as defined by the Roche 2nd Derivative Algorithm calling a positive identification). When concentration was reduced to 100 copies per mL of wastewater effluent (2.5 copies per PCR reaction), we detected SARS-CoV-2 in six of eight replicates, which is predicted by the Poisson distribution of the RNA spike (i.e., some PCR reactions will have zero copies at this dilution). This “digital PCR-like” readout warrants some additional discussion, as our high replicate number addresses sample heterogeneity. In other words, we combined eight replicate measurements into a single readout, rather than reporting eight independent readouts, to more reliably identify a positive aliquot within a large and heterogeneous sample. Thus, using conservative criteria, we conclude that our LoD was approximately 1000 copies per mL. However, as our technology enables several replicates to be performed in parallel, we observed that samples containing 100 copies per mL could be detected in the majority (6 of 8) of replicates and even lower SARS-CoV-2 RNA concentrations could be identified by 1–2 positive replicates. This reduced detection was likely a combination of wastewater heterogeneity and Poisson distribution of SARS-CoV-2 RNA per replicate (Figure 4B). In summary, our assay was able to reliably detect heat-inactivated SARS-CoV-2 in samples containing <100 copies per mL, but confidence in results increased as concentration increased up to 1000 copies per mL. For comparison, we also independently measured the limit of detection of the RT-qPCR assay alone using RNA from heat-inactivated SARS-CoV-2 (BEI NR-52285) titrated in nuclease free water. We found that 50 copies per reaction was detected in 3/3 replicates, 5 copies per reaction was detected in 2/3 replicates, and 0.5 copies per reaction was detected in 1/3 replicates. Additionally, room temperature ESP-extracted aliquots of SARS-CoV-2 RNA maintained a high SARS-CoV-2 signal longer than samples stored at 4°C or room temperature (see Supplementary Table S1), as with our prior HIV work25
Residence Hall Wastewater Surveillance and Correlation with Clinical Data
During two semesters of residence hall wastewater surveillance, 129 wastewater samples drawn from both individual residence halls and hall clusters had an overall positivity rate (i.e., positive samples / total samples) of 34%. Two of five residence hall clusters (D and E) returned only one positive wastewater signal during the semester and were therefore excluded from subsequent analysis. Residence Hall D had significantly different layout with independent apartment entrances and limited shared space. Residence Hall E had a significantly lower and more transient population. These characteristics may explain the markedly lower wastewater positivity at these locations. Wastewater samples from the remaining three hall clusters had a positivity of 56%. Comparisons of wastewater measurements and clinical test positivity (defined as the ratio of positive clinical tests to total clinical tests) for residence hall clusters A through C are shown in Figure 5A–C, respectively. Given that infected individuals variably shed virus26–28 (variability exists in both quantity and timeframe), a quantitative comparison of wastewater and clinical test positivity may have limited utility for small numbers of individuals (an average of 1.9 clinically positive individuals per cluster per week among Clusters A-C). Therefore, we also qualitatively compared clinical testing and dormitory wastewater analysis results for each week (Monday to Monday) (Figure 5D). Out of 45 qualitative comparisons (3 clusters over 15 weeks), 27 were in agreement.
Figure 5:
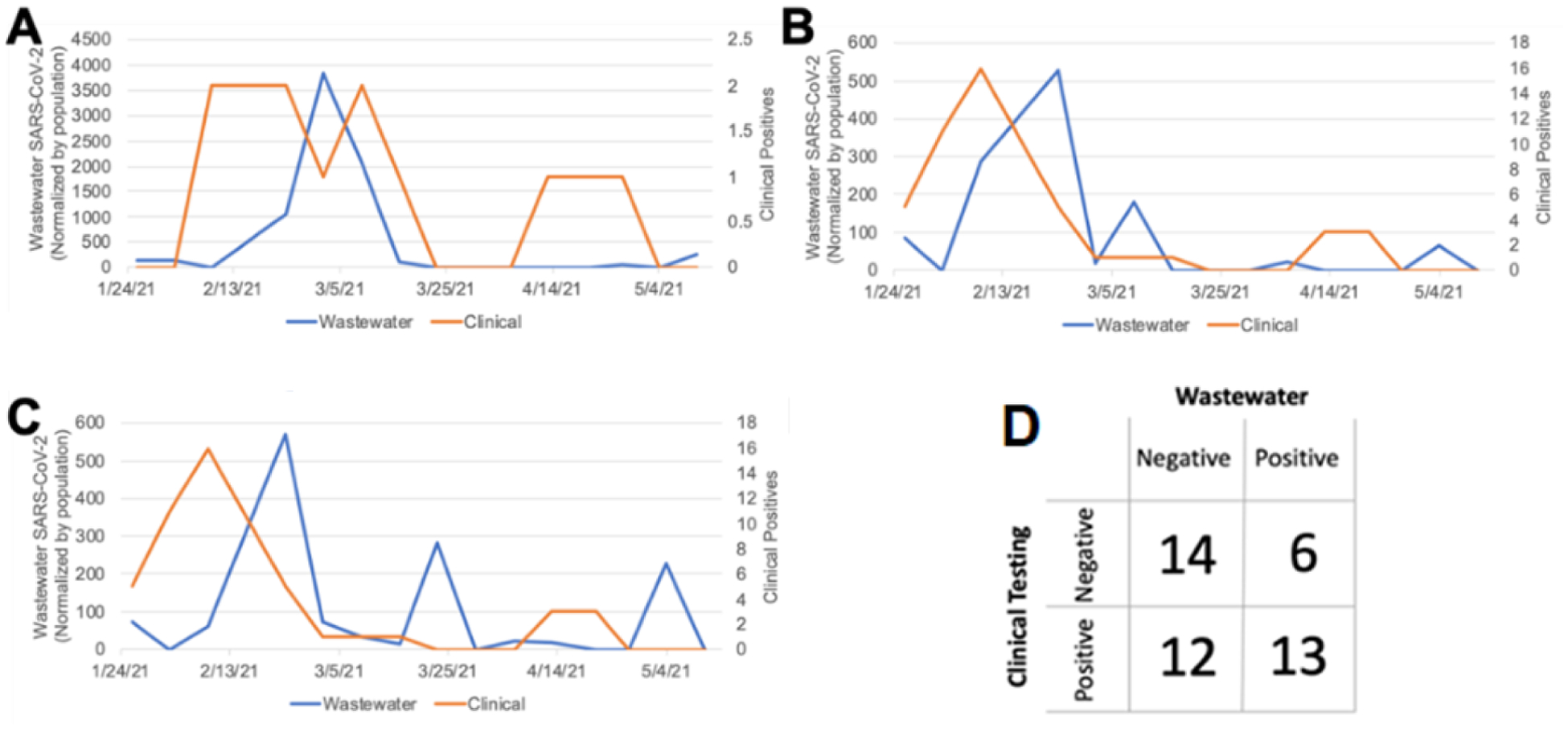
The relationship between wastewater SARS-CoV-2 RNA concentration (left-sided y-axis, blue line) and PCR-confirmed student SARS-CoV-2 infections (right-sided y-axis, orange line) during spring semester 2021 at residence hall clusters A-C. Note that the scales of the y-axes differ between graphs A-C. D) Correlation between wastewater measurements and the subsequent week’s clinical testing.
In six instances, no clinical positives were observed in the week following a positive wastewater signal. One of these six “false positive” instances occurred during the first week of the spring semester when residence hall move-in was in progress. During this week, contributions to the wastewater may have included individuals (e.g., parents) not living in the residence hall. The other five “false positive” wastewater results occurred during weeks with low clinical testing rates for the respective residence hall cluster (an average of 4% of residents tested across these five instances compared with an average of 15% for the complete study period). Positive individuals may have gone clinically undetected during these weeks given the relatively low clinical testing coverage. No consistent trends were observed to explain 12 instances of wastewater-negative, clinical-positive results. These “false negative” events may be related to inherent limitations of wastewater testing as addressed in the Discussion Section.
When autosampler availability allowed, detections of high wastewater positivity (typically >200 cp/mL, depending on autosampler availability) in residence hall cluster sewer-sheds triggered more granular follow-up sampling. Follow up sampling targeted effluent pipes of individual residence halls (as illustrated in Figure 2), enabling the UK Emergency Operation Center (EOC) to identify hotspots and consider additional clinical testing. In 12 of 14 total follow-up events, wastewater for at least one hall within the identified cluster produced a positive signal. Additional clinical testing occurred after eight of these events, successfully identifying previously undetected infections in five instances. In one example, after measuring an unusually high level of SARS-CoV-2 in wastewater (4,537 cp/mL) at a residence hall in November 2020, enhanced clinical testing identified 15 positive students over the subsequent week, nearly twice the number identified during any other seven-day period in the fall at the same residence hall.
Discussion
Wastewater surveillance presents unique challenges in data interpretation. Unlike emerging clinical tests that have a “gold standard” for benchmarking, wastewater testing of SARS-CoV-2 does not. It often involves the correlation of an inherently noisy measurement (wastewater) with incomplete clinical data, like the results of testing students in University of Kentucky residence halls. Indeed, wastewater surveillance and clinical testing both suffer from limitations that mitigate the value of direct comparison between these two methods. For example, approximately half of SARS-CoV-2 infections are asymptomatic.29 Consequently, it is likely that many SARS-CoV-2 infected students were never tested, potentially explaining the presence of positive wastewater signals in the absence of identified clinical infections.
Fecal shedding is known to be highly variable across patients30. The association between wastewater surveillance and clinical testing is further convoluted by the fecal contributions of convalescent shedders. Recently recovered students – who were likely still shedding SARS-CoV-2 RNA into the sewer26 – may have been responsible for a positive wastewater result while being clinically “undetectable.” The effect of convalescent shedding may also explain why wastewater spikes were often observed ~2 weeks following positive clinical test results (Figure 5B–C). Specifically, following a positive test, students were relocated to an isolation residence hall for 10–14 days but subsequently returned to their original residence hall, at which point they may have resumed shedding into the residence hall under surveillance.
Discordance between wastewater data and clinical data may also have stemmed from infected individuals who simply did not contribute bowel movements during autosampler collection windows. Alternatively, they may not have been shedding virus in their feces at all26. These scenarios may explain the presence of positive clinical results in the absence of a positive wastewater signal.
Incomplete clinical testing, variable shedding rates, and sheer chance make apparent “false positives” and “false negatives” in wastewater surveillance data difficult to verify. In summary, a significant challenge facing development of wastewater assays is the lack of a true “apples to apples” comparator against which to benchmark performance. However, wastewater surveillance has the potential to catch outbreaks in their early stages31 and is an efficient method for assessing infection trends in populations. Unfortunately, this powerful tool is limited by its reliance upon advanced molecular testing facilities and the ability to rapidly ship samples while maintaining a robust cold chain.
Conclusion
Here, we present a new analytical method to quickly and affordably concentrate, extract, and analyze SARS-CoV-2 in wastewater samples. Our novel ESP approach to wastewater surveillance is fast (70% reduction in time compared to traditional PMP extraction protocols) and requires fewer supplies (40% reduction in consumables compared to traditional PMP extraction protocols) and personnel than its more traditional counterparts. While our concentration factor is relatively modest (2.5-fold), it is combined with relatively high efficiency (average of 89%), resulting in an LoD sufficient to regularly detect a relatively modest number of clinical cases (an average of 1.9 clinically positive students per residence hall per week). Further, we eliminate a separate laborious pre-concentration step (e.g., ultracentrifugation, ultrafiltration, or flocculation) that is time- and resource-intensive and exhibited variable reproducibility in prior studies32–34.
In conclusion, the wastewater surveillance method presented in this paper combines accurate analytical performance with a significant reduction in operational complexity. Further, this method represents a means to “field stabilize” labile RNA biomarkers via extraction as the rapid and variable decay rates of SARS-CoV-2 RNA in wastewater compromise analytical sensitivity and precision35. We anticipate that these laboratory advances will improve the accessibility of wastewater surveillance, thus enabling implementation in regions that currently lack access to this powerful surveillance tool.
Supplementary Material
Acknowledgements
The following reagent was deposited by the Centers for Disease Control and Prevention and obtained through BEI Resources, NIAID, NIH: Genomic RNA from SARS-Related Coronavirus 2, Isolate USA-WA1/2020, NR-52285.
This work was funded by National Institutes of Health (NIH) grants 1U01DA053903-01 and P30 ES026529, Centers for Disease Control and Prevention (CDC) contract BAA 75D301-20-R-68024. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or CDC.
The authors would like to thank Blazan Mijatovic, Cullen Olsson, and Savannah Tucker for their aid in collecting wastewater samples and refining the extraction protocol.
Footnotes
Supplementary Information
Additional experimental methods and results, including data regarding degradation of SARS-CoV-2 and details concerning the optimization of wastewater sampling cadence.
Disclosures
S.B. has an ownership interest in Salus Discovery, LLC, which has licensed the ESP technology described in the text.
References
- (1).Murakami M; Hata A; Honda R; Watanabe T Letter to the Editor: Wastewater-Based Epidemiology Can Overcome Representativeness and Stigma Issues Related to COVID-19. Environ. Sci. Technol 2020. 10.1021/acs.est.0c02172. [DOI] [PubMed] [Google Scholar]
- (2).Chen Y; Chen L; Deng Q; Zhang G; Wu K; Ni L; Yang Y; Liu B; Wang W; Wei C; Yang J; Ye G; Cheng Z The Presence of SARS-CoV-2 RNA in the Feces of COVID-19 Patients. J. Med. Virol 2020, 92 (7), 833–840. 10.1002/jmv.25825. [DOI] [PubMed] [Google Scholar]
- (3).Carducci A; Federigi I; Dasheng L; T. Julian R; Marco V Making Waves: Coronavirus Detection, Presence and Persistence in the Water Environment: State of the Art and Knowledge Needs for Public Health. Water Research Elsevier Ltd; July 15, 2020. 10.1016/j.watres.2020.115907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Wurtzer S; Marechal V; Mouchel J-M; Moulin L Time Course Quantitative Detection of SARS-CoV-2 in Parisian Wastewaters Correlates with COVID-19 Confirmed Cases. medRxiv 2020, 2020.04.12.20062679. 10.1101/2020.04.12.20062679. [DOI] [Google Scholar]
- (5).Ahmed W; Angel N; Edson J; Bibby K; Bivins A; O’Brien JW; Choi PM; Kitajima M; Simpson SL; Li J; Tscharke B; Verhagen R; Smith WJM; Zaugg J; Dierens L; Hugenholtz P; Thomas KV; Mueller JF First Confirmed Detection of SARS-CoV-2 in Untreated Wastewater in Australia: A Proof of Concept for the Wastewater Surveillance of COVID-19 in the Community. Sci. Total Environ 2020, 138764. 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Gibas C; Lambirth K; Mittal N; Juel MAI; Barua VB; Roppolo Brazell L; Hinton K; Lontai J; Stark N; Young I; Quach C; Russ M; Kauer J; Nicolosi B; Chen D; Akella S; Tang W; Schlueter J; Munir M Implementing Building-Level SARS-CoV-2 Wastewater Surveillance on a University Campus. Sci. Total Environ 2021, 782, 146749. 10.1016/j.scitotenv.2021.146749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).D’Aoust PM; Graber TE; Mercier E; Montpetit D; Alexandrov I; Neault N; Baig AT; Mayne J; Zhang X; Alain T; Servos MR; Srikanthan N; MacKenzie M; Figeys D; Manuel D; Jüni P; MacKenzie AE; Delatolla R Catching a Resurgence: Increase in SARS-CoV-2 Viral RNA Identified in Wastewater 48 h before COVID-19 Clinical Tests and 96 h before Hospitalizations. Sci. Total Environ 2021, 770, 145319. 10.1016/j.scitotenv.2021.145319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Medema G; Heijnen L; Elsinga G; Italiaander R; Brouwer A Presence of SARS-Coronavirus-2 RNA in Sewage and Correlation with Reported COVID-19 Prevalence in the Early Stage of the Epidemic in The Netherlands. Environ. Sci. Technol. Lett 2020, 7 (7), 511–516. 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- (9).Davó L; Seguí R; Botija P; Beltrán MJ; Albert E; Torres I; López-Fernández PÁ; Ortí R; Maestre JF; Sánchez G; Navarro D Early Detection of SARS-CoV-2 Infection Cases or Outbreaks at Nursing Homes by Targeted Wastewater Tracking. Clin. Microbiol. Infect 2021, 27 (7), 1061–1063. 10.1016/j.cmi.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Kirby AE Using Wastewater Surveillance Data to Support the COVID-19 Response — United States, 2020–2021. MMWR Morb. Mortal. Wkly. Rep 2021, 70. 10.15585/mmwr.mm7036a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Betancourt WQ; Schmitz BW; Innes GK; Prasek SM; Pogreba Brown KM; Stark ER; Foster AR; Sprissler RS; Harris DT; Sherchan SP; Gerba CP; Pepper IL COVID-19 Containment on a College Campus via Wastewater-Based Epidemiology, Targeted Clinical Testing and an Intervention. Sci. Total Environ 2021, 779, 146408. 10.1016/j.scitotenv.2021.146408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Corchis-Scott R; Geng Q; Seth R; Ray R; Beg M; Biswas N; Charron L; Drouillard KD; D’Souza R; Heath DD; Houser C; Lawal F; McGinlay J; Menard SL; Porter LA; Rawlings D; Scholl ML; Siu KWM; Tong Y; Weisener CG; Wilhelm SW; McKay RML Averting an Outbreak of SARS-CoV-2 in a University Residence Hall through Wastewater Surveillance. Microbiol. Spectr 2021, 9 (2), e0079221. 10.1128/Spectrum.00792-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Hart OE; Halden RU Computational Analysis of SARS-CoV-2/COVID-19 Surveillance by Wastewater-Based Epidemiology Locally and Globally: Feasibility, Economy, Opportunities and Challenges. Sci. Total Environ 2020, 730, 138875. 10.1016/j.scitotenv.2020.138875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Berry SM; Pezzi HM; Williams ED; Loeb JM; Guckenberger DJ; LaVanway AJ; Puchalski AA; Kityo CM; Mugyenyi PN; Graziano FM; Beebe DJ Using Exclusion-Based Sample Preparation (ESP) to Reduce Viral Load Assay Cost. PLoS ONE 2015, 10 (12). 10.1371/journal.pone.0143631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Berry SM; Chin EN; Jackson SS; Strotman LN; Goel M; Thompson NE; Alexander CM; Miyamoto S; Burgess RR; Beebe DJ Weak Protein-Protein Interactions Revealed by Immiscible Filtration Assisted by Surface Tension. Anal. Biochem 2014, 447, 133–140. 10.1016/j.ab.2013.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Berry SM; Alarid ET; Beebe DJ One-Step Purification of Nucleic Acid for Gene Expression Analysis via Immiscible Filtration Assisted by Surface Tension (IFAST). Lab. Chip 2011, 11 (10), 1747–1753. 10.1039/c1lc00004g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Berry SM; Singh C; Lang JD; Strotman LN; Alarid ET; Beebe DJ Streamlining Gene Expression Analysis: Integration of Co-Culture and MRNA Purification. Integr. Biol. Quant. Biosci. Nano Macro 2014, 6 (2), 224–231. 10.1039/c3ib40136g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Strotman LN; Lin G; Berry SM; Johnson EA; Beebe DJ Facile and Rapid DNA Extraction and Purification from Food Matrices Using IFAST (Immiscible Filtration Assisted by Surface Tension). The Analyst 2012, 137 (17), 4023–4028. 10.1039/c2an35506j. [DOI] [PubMed] [Google Scholar]
- (19).Moussavi-Harami SF; Annis DS; Ma W; Berry SM; Coughlin EE; Strotman LN; Maurer LM; Westphall MS; Coon JJ; Mosher DF; Beebe DJ Characterization of Molecules Binding to the 70K N-Terminal Region of Fibronectin by IFAST Purification Coupled with Mass Spectrometry. J. Proteome Res 2013, 12 (7), 3393–3404. 10.1021/pr400225p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Pezzi HM; Niles DJ; Schehr JL; Beebe DJ; Lang JM Integration of Magnetic Bead-Based Cell Selection into Complex Isolations. ACS Omega 2018, 3 (4), 3908–3917. 10.1021/acsomega.7b01427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Batéjat C; Grassin Q; Manuguerra J-C; Leclercq I Heat Inactivation of the Severe Acute Respiratory Syndrome Coronavirus 2. J. Biosaf. Biosecurity 2021, 3 (1), 1–3. 10.1016/j.jobb.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Robinson CA; Hsieh H-Y; Hsu S-Y; Wang Y; Salcedo BT; Belenchia A; Klutts J; Zemmer S; Reynolds M; Semkiw E; Foley T; Wan X; Wieberg CG; Wenzel J; Lin C-H; Johnson MC Defining Biological and Biophysical Properties of SARS-CoV-2 Genetic Material in Wastewater. Sci. Total Environ 2022, 807 (Pt 1), 150786. 10.1016/j.scitotenv.2021.150786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Escobar MD; Hunt JL A Cost-Effective RNA Extraction Technique from Animal Cells and Tissue Using Silica Columns. J. Biol. Methods 2017, 4 (2). 10.14440/jbm.2017.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Centers of Disease Control and Prevention. 2019-Novel Coronavirus (2019-NCoV) Real-Time RRT-PCR Panel Primers and Probes May 29, 2020.
- (25).Berry SM; Pezzi HM; Williams ED; Loeb JM; Guckenberger DJ; Lavanway AJ; Puchalski AA; Kityo CM; Mugyenyi PN; Graziano FM; Beebe DJ Using Exclusion-Based Sample Preparation (ESP) to Reduce Viral Load Assay Cost. PLOS ONE 2015, 10 (12), e0143631. 10.1371/journal.pone.0143631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Wu Y; Guo C; Tang L; Hong Z; Zhou J; Dong X; Yin H; Xiao Q; Tang Y; Qu X; Kuang L; Fang X; Mishra N; Lu J; Shan H; Jiang G; Huang X Prolonged Presence of SARS-CoV-2 Viral RNA in Faecal Samples. Lancet Gastroenterol. Hepatol 2020, 5 (5), 434–435. 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Zheng S; Fan J; Yu F; Feng B; Lou B; Zou Q; Xie G; Lin S; Wang R; Yang X; Chen W; Wang Q; Zhang D; Liu Y; Gong R; Ma Z; Lu S; Xiao Y; Gu Y; Zhang J; Yao H; Xu K; Lu X; Wei G; Zhou J; Fang Q; Cai H; Qiu Y; Sheng J; Chen Y; Liang T Viral Load Dynamics and Disease Severity in Patients Infected with SARS-CoV-2 in Zhejiang Province, China, January-March 2020: Retrospective Cohort Study. BMJ 2020, 369, m1443. 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Wölfel R; Corman VM; Guggemos W; Seilmaier M; Zange S; Müller MA; Niemeyer D; Jones TC; Vollmar P; Rothe C; Hoelscher M; Bleicker T; Brünink S; Schneider J; Ehmann R; Zwirglmaier K; Drosten C; Wendtner C Virological Assessment of Hospitalized Patients with COVID-2019. Nature 2020, 581 (7809), 465–469. 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- (29).Oran DP; Topol EJ The Proportion of SARS-CoV-2 Infections That Are Asymptomatic. Ann. Intern. Med 2021, M20–6976. 10.7326/M20-6976. [DOI] [PubMed] [Google Scholar]
- (30).Foladori P; Cutrupi F; Segata N; Manara S; Pinto F; Malpei F; Bruni L; La Rosa G SARS-CoV-2 from Faeces to Wastewater Treatment: What Do We Know? A Review. Sci. Total Environ 2020, 743, 140444. 10.1016/j.scitotenv.2020.140444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Driver EM; Gushgari A; Chen J; Halden RU Alcohol, Nicotine, and Caffeine Consumption on a Public U.S. University Campus Determined by Wastewater-Based Epidemiology. Sci. Total Environ 2020, 727, 138492. 10.1016/j.scitotenv.2020.138492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Ahmed W; Bertsch PM; Bivins A; Bibby K; Farkas K; Gathercole A; Haramoto E; Gyawali P; Korajkic A; McMinn BR; Mueller JF; Simpson SL; Smith WJM; Symonds EM; Thomas KV; Verhagen R; Kitajima M Comparison of Virus Concentration Methods for the RT-qPCR-Based Recovery of Murine Hepatitis Virus, a Surrogate for SARS-CoV-2 from Untreated Wastewater. Sci. Total Environ 2020, 739, 139960. 10.1016/j.scitotenv.2020.139960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Zheng X; Deng Y; Xu X; Li S; Zhang Y; Ding J; On HY; Lai JCC; In Yau C; Chin AWH; Poon LLM; Tun HM; Zhang T Comparison of Virus Concentration Methods and RNA Extraction Methods for SARS-CoV-2 Wastewater Surveillance. Sci. Total Environ 2022, 824, 153687. 10.1016/j.scitotenv.2022.153687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Barril PA; Pianciola LA; Mazzeo M; Ousset MJ; Jaureguiberry MV; Alessandrello M; Sánchez G; Oteiza JM Evaluation of Viral Concentration Methods for SARS-CoV-2 Recovery from Wastewaters. Sci. Total Environ 2021, 756, 144105. 10.1016/j.scitotenv.2020.144105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Bivins A; Greaves J; Fischer R; Yinda KC; Ahmed W; Kitajima M; Munster VJ; Bibby K Persistence of SARS-CoV-2 in Water and Wastewater. Environ. Sci. Technol. Lett 2020, acs.estlett.0c00730. 10.1021/acs.estlett.0c00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Philo SE; Keim EK; Swanstrom R; Ong AQW; Burnor EA; Kossik AL; Harrison JC; Demeke BA; Zhou NA; Beck NK; Shirai JH; Meschke JS A Comparison of SARS-CoV-2 Wastewater Concentration Methods for Environmental Surveillance. Sci. Total Environ 2021, 760, 144215. 10.1016/j.scitotenv.2020.144215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Wilder ML; Middleton F; Larsen DA; Du Q; Fenty A; Zeng T; Insaf T; Kilaru P; Collins M; Kmush B; Green HC Co-Quantification of CrAssphage Increases Confidence in Wastewater-Based Epidemiology for SARS-CoV-2 in Low Prevalence Areas. Water Res. X 2021, 11, 100100. 10.1016/j.wroa.2021.100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Rusiñol M; Martínez-Puchol S; Forés E; Itarte M; Girones R; Bofill-Mas S Concentration Methods for the Quantification of Coronavirus and Other Potentially Pandemic Enveloped Virus from Wastewater. Curr. Opin. Environ. Sci. Health 2020, 17, 21–28. 10.1016/j.coesh.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


