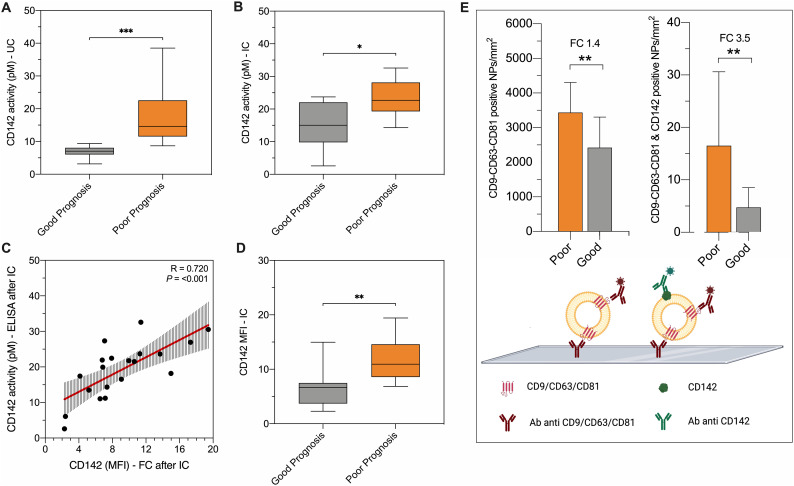
Fig. 4.
Experimental validation with different techniques.
The discriminant performance of CD142-EV was experimentally validated by different techniques in patients with SARS-CoV2 infection: good prognosis (grey; n = 10) vs. poor prognosis (orange; n = 10). (A-B) CD142 activity per particle measured by ELISA (pM per 109 particles), after EV isolation by ultracentrifugation (UC) or immunocapture (IC using beads covered by antibodies against CD9-CD63-CD81). (C) Correlation between CD142 activity per particle (pM) and CD142 MFI at flow cytometry after IC. (D) CD142-EV MFI after IC (direct staining after immuno-capture, using beads covered by antibodies against CD9-CD63-CD81). (E) Colocalization of tetraspanins (CD9-CD63-CD81) and CD142 was assessed by ExoView® R100 Analyzer. Data are reported for mean number of nanoparticles (NPs) per mm2 for vesicles labelled with fluorochrome-conjugated antibodies against CD9-CD63-CD81 and for the double positive for CD9-CD63-CD81 and CD142.