Fig. 5.
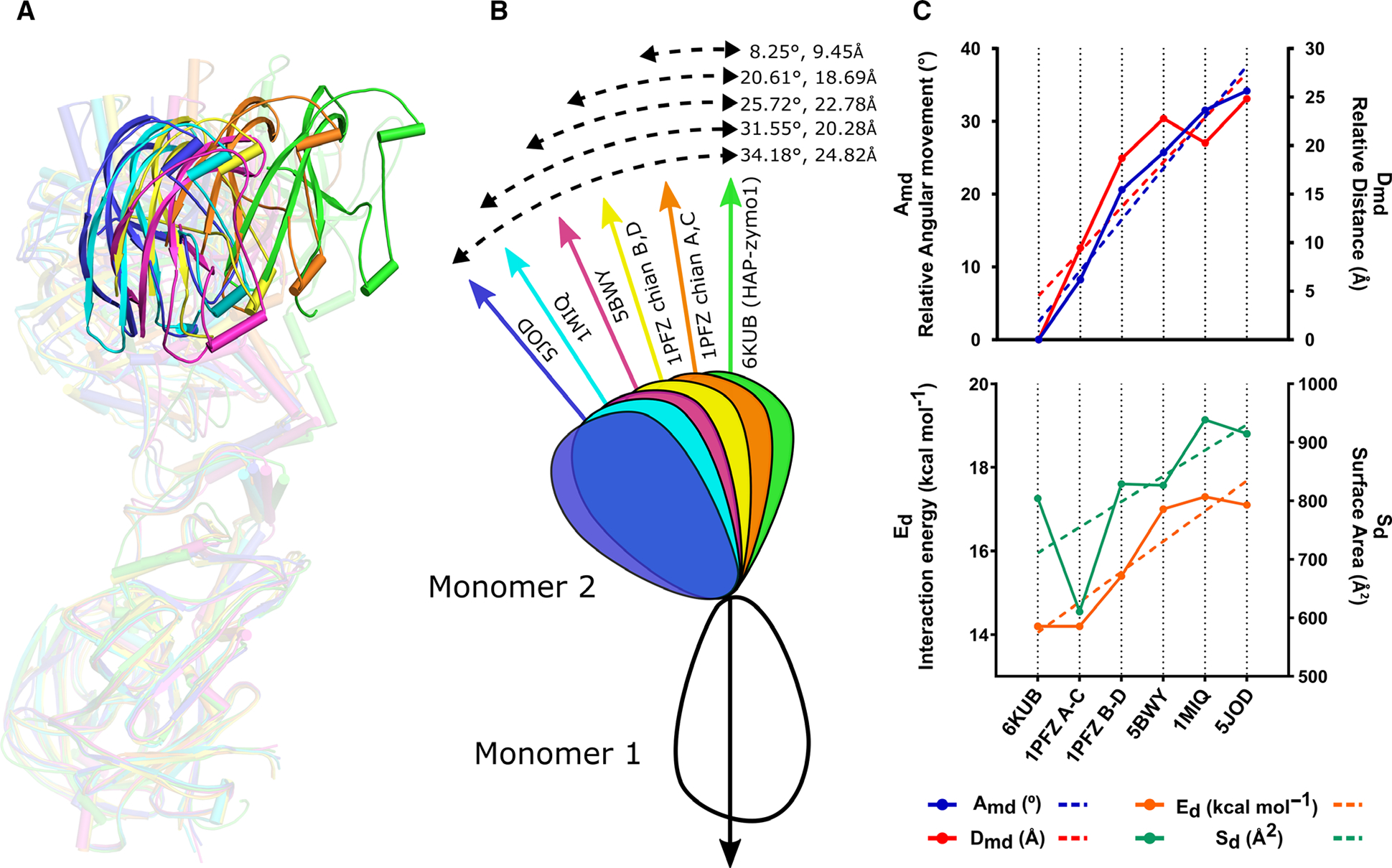
Comparison of the relative orientations of the monomers in the dimeric structures of pro-tPMs. (A) Superposition of the dimeric forms of HAP-zymo1 (green; 6KUB) with pro-tPMII (1PFZ; chain A and C in orange), pro-tPMII (1PFZ; chain B and D in yellow), pro-tPMII (5BWY in magenta), Pv-pro-tPM (1MIQ in cyan), and pro-tPMIV (5JOD in blue) with respect to monomer 1. The transparency of the dimers is increased to highlight the relative movement of the structures. The protein structural representations have been generated with the PyMOL molecular graphics system (Schrodinger LLC, New York, NY, USA; version 2.3.2). (B) Schematic representation of the superposed dimers of pro-tPMs with respect to HAP-zymo1 (6KUB) and the relative movements (angle and distance) observed. (C) Graphs representing the variation of angle (Amd) and distance (Dmd) in the structures of pro-tPMs (upper panel); energy of interaction (Ed) and the area enclosed in the dimers (Sd) of pro-tPMs (lower panel).
