Fig. 9.
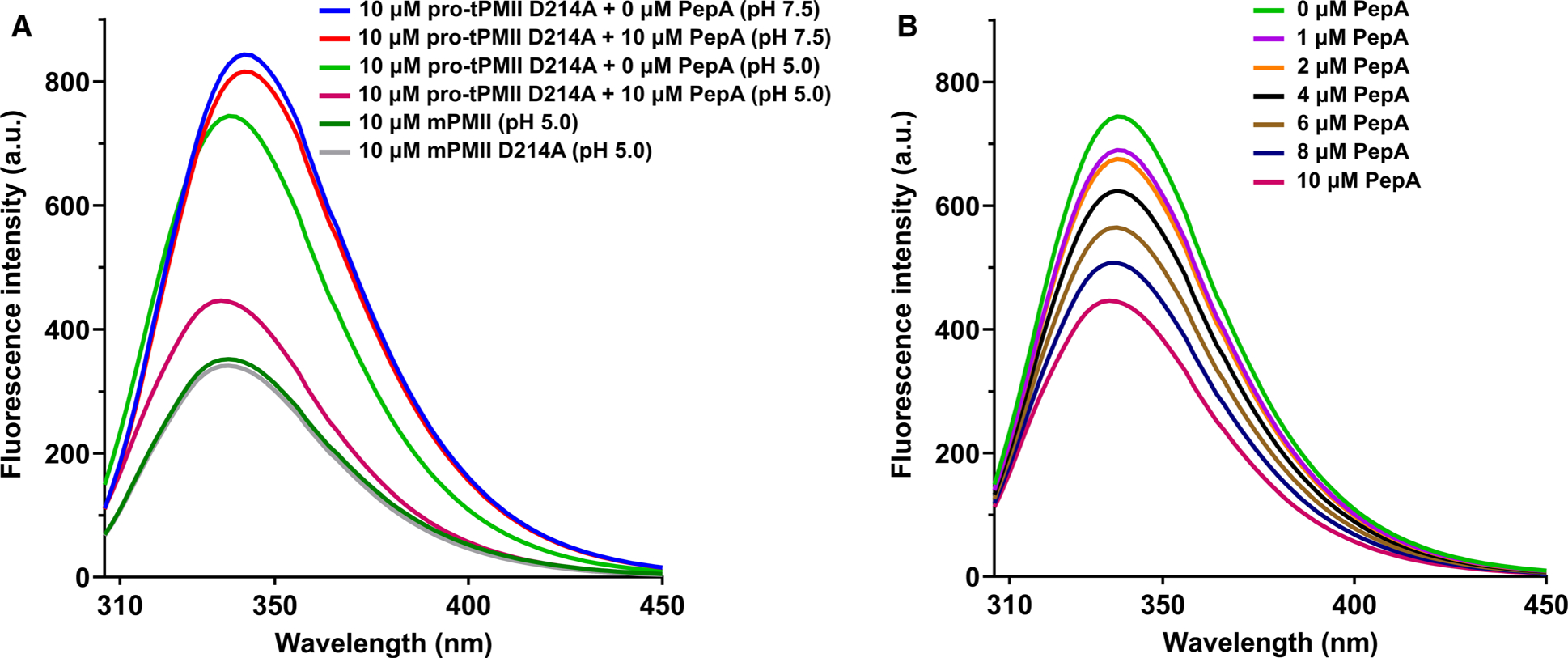
Tryptophan fluorescence quenching of pro-tPMII D214A mutant. (A) Quenching of fluorescence of pro-tPMII D214A in pH 7.5 and 5.0, with and without inhibitor; mature PMII and mature PMII D214A mutant are taken as positive controls. (B) Fluorescence quenching of pro-tPMII D214A mutant at pH 5.0 in the presence of PepA (0–10 μm). The experiments were performed in quintuplicate (n = 5), and the data are represented as a mean of all the measurements.
