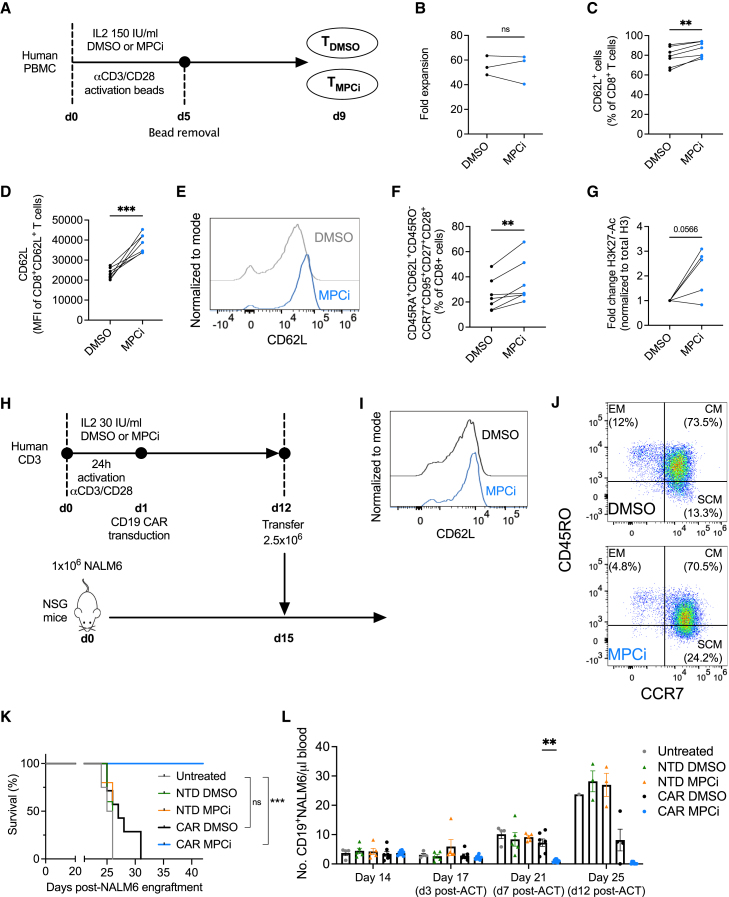
Figure 7.
MPC inhibition dramatically improves human CD19-CAR T cell therapy in a xenograft leukemia model
(A) Experimental scheme.
(B) Fold expansion after 9 days of culture (n = 3 human donors/group).
(C–E) Percentage CD62L-positive CD8+ T cells (C) and median fluorescent intensity (MFI) of CD62L in the CD62L-positive population (D), and representative histogram from one donor (E).
(F) Percentage of stem cell-like memory CD8+ T cells, measured by flow cytometry.
In (C)–(F), n = 7 human donors/group; pooled data from 2 independent experiments.
(G) Western blot quantification of H3K27 acetylation shown as fold change compared with DMSO (n = 5 human donors/group; pooled data from 2 independent experiments).
(H) Experimental scheme.
(I and J) Histogram of CD62L expression (I) and FACS plot showing CD45-RO and CCR7 expression (J) in CD8+ T cells from a representative donor, 7 days after transduction (EM, effector memory; CM, central memory; SCM, stem cell-like memory).
(K) Overall survival.
(L) Number of NALM6 cells in the blood (n = 4 mice for untreated, n = 5 mice for NTD DMSO and NTD MPCi, n = 7 mice for CAR DMSO, and n = 8 mice for CAR MPCi; pooled data from 2 independent experiments).
Data are represented as mean ± SEM. Statistics are based on paired, two-tailed Student’s t test (B–D and F–G), log rank test (K), or two-way ANOVA using Fisher’s LSD test (L), ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, or as indicated. See also Figure S7.