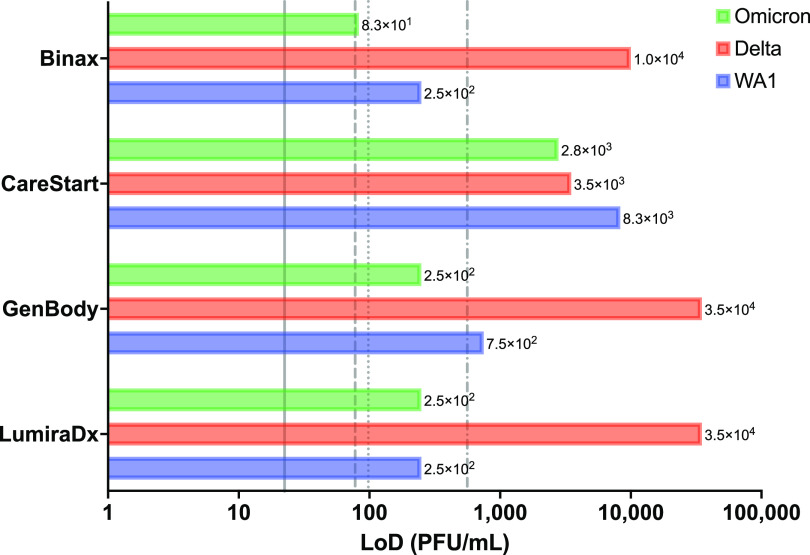
FIG 1.
Limits of detection of antigen tests. Shown are the limits of detection (LoDs) in PFU per milliliter determined in our analysis (bars). Vertical lines reference the manufacturer-reported LoDs in the respective instructions for use (IFU) documents (11–14), converted from 50% tissue culture infective doses (TCID50) per milliliter to PFU per milliliter by multiplying the TCID50 per milliliter by 0.7, a standard conversion based on the Poisson distribution, for BinaxNOW (dotted line) (1.4 × 102 TCID50/mL; 9.8 × 101 PFU/mL), CareStart (dashed and dotted line) (8.0 × 102 TCID50/mL; 5.6 × 102 PFU/mL), GenBody (dashed line) (1.1 × 102 TCID50/mL; 7.8 × 101 PFU/mL), and LumiraDx (solid line) (3.2 × 101 TCID50/mL; 2.2 × 101 PFU/mL).