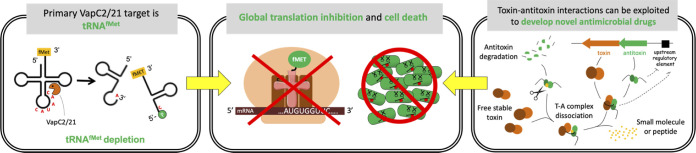
FIG 7.
Summary of proposed VapC2 and VapC21 mechanism of action and application as targets for antitubercular development. VapC2 and VapC21 specifically cleave and inactivate initiator tRNAfMet (left panel). Sustained toxin expression leads to depletion of this tRNA which is essential for translation of M. tuberculosis mRNAs, resulting in cell death (center panel). These properties can be harnessed for drug development as illustrated in the right panel. TA modules typically reside in an autoregulated operon (repressed by antitoxin alone or the TA complex); the antitoxin gene precedes the toxin gene (top right of right panel). After their transcription and translation, the antitoxin protein is intrinsically unfolded (and thus more susceptible to proteolytic cleavage) until it assembles with its cognate antitoxin. Identification of small-molecular inhibitors (yellow) of the T-A interaction is predicted to drive this dynamic system toward excess, free toxin and cell death because free antitoxin with bound inhibitor will be susceptible to degradation by M. tuberculosis proteases (scissors).