FIG 8.
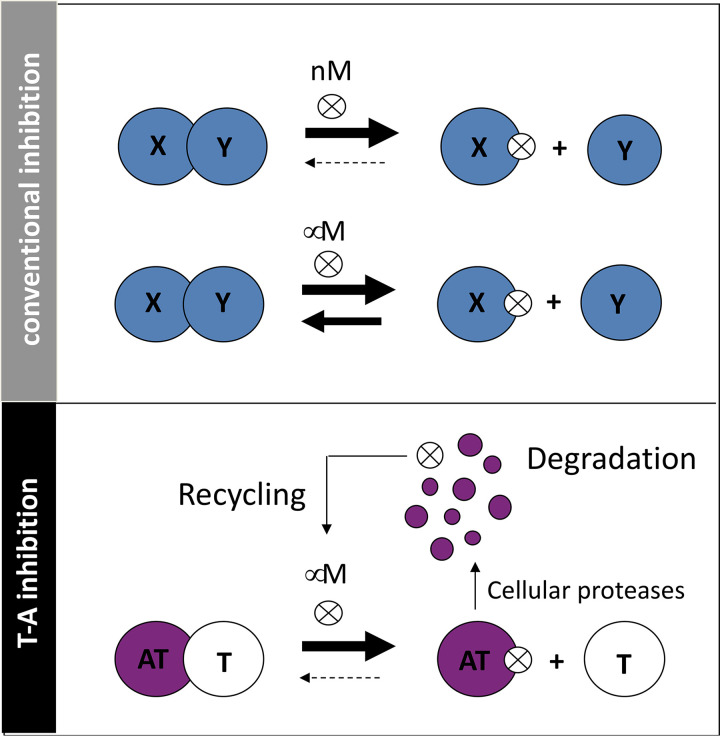
Small-molecular inhibitors of T-A association are predicted to be effective at lower affinities than conventional protein complexes. (Top) In conventional inhibition of protein-protein interaction with small molecules or peptides (⊗), it is most desirable to select those that bind at nanomolar affinities to favor maintenance of the dissociated complex; micromolar affinities are often not potent enough to keep the proteins dissociated. (Below) In contrast, for TA complexes the intrinsic instability of the antitoxin coupled with inhibitor recycling upon antitoxin degradation is predicted to potentiate the efficacy of inhibitors; micromolar affinities may be sufficient to favor stable dissociation of the two proteins.