Abstract
Introduction
Lately, immunotherapy has evolved as a safe and reliable management option for treatment of warts. Various immunogens among others in use include vaccines and antigens like purified Protein Derivative of Tuberculin (PPD) and mycobacterium w (Mw) or mycobacterium indicum prani vaccines.
Objectives
The study was aimed to assess the effectiveness and safety profile of intralesional Mw vaccine against intralesional PPD for the management of multiple warts with assessment of the improvement in quality of life (QoL) using the Dermatology Life Quality Index questionnaire.
Methods
Patients aged above 12 years with ≥2 warts were recruited for the study. These individuals were randomized into groups A and B, namely Mw vaccine (group A) and PPD Tuberculin (group B). At each visit, 0.1–0.2 ml of active antigen was infiltrated intralesionally into the largest/mother wart. The injections were repeated after every 4 weeks, for the next 12 weeks. QoL improvement was measured.
Results
This intention to treat analysis was completed by 102 patients, of which 55 were in group A and 47 in group B. The rate of complete clearance was comparable in group A (76.3%) with the one in group B (65.9%, P = 0.064). Prior to treatment initiation, the most severely impacted domain of life by warts was ‘symptoms and feelings’. There was a statistically significant improvement in QoL at the end of the treatment ( P <0.01).
Conclusion
Mw vaccine holds leverage over PPD with a marginally higher rate of clearance and less adverse events for managing warts.
Keywords: warts, HPV, PPD tuberculin, immuvac vaccine, Mw vaccine, intralesional immunogens
Introduction
Warts or verruca are benign tumors cause by the infection of keratinocytes with the Human Papilloma Virus (HPV) [1]. The prevalence rates of warts range from 3%–20%, with a gradual increase in incidence rate from childhood to adolescence, followed by a rapid decline after the late 20s [2]. Even though in most of the cases these warts resolve on their own, the patients often resort to various treatment options due to their unsightly appearance, symptomatology and potentially contagious nature.
Traditionally, used destructive therapies for warts include surgical excision, cryotherapy, radiofrequency ablation, electrical and chemical cauterization, etc. Bleomycin, salicylic acid, trichloroacetic acid, podophyllin, 5-fluorouracil, imiquimod, are a few of the conventional pharmacological agents in use. Over the last few decades, even lasers and photodynamic therapy have been employed to treat warts. Nevertheless, the usefulness of all these therapies is limited due to their absence of antiviral properties, high recurrence rates, and the need to treat ever wart individually [2].
Various intralesional antigens that have been utilized for warts, namely, Bacillus Calmette and Guerin (BCG) vaccine, measles, mumps, and rubella (MMR) vaccine, Mycobacterium w (Mw) vaccine, skin test antigens like purified protein derivative of tuberculin (PPD), mumps, trichophyton, and Candida with good success rates [3,4].
Objectives
This prospective, randomized open label study was aimed to assess the effectiveness and safety profile of intralesional Mw vaccine against intralesional PPD for the management of multiple warts and improvement in patients’ quality of life (QoL).
Methods
Study Design and Randomization
This was a prospective, parallel-group study conducted at the dermatology out-patient department of our tertiary care hospital. Patients with 2 or more extragenital warts were evaluated and recruited for our study into two arms, namely group A (Mw vaccine) and group B (PPD tuberculin). Demographic data and baseline parameters including number and site of warts were noted. The patients were randomized by asking them to choose a number from a simple random number table. However, since this study was done in an open-label setting, both the patients and the investigators were aware of the treatment being received. The study was approved by our institutional ethical board (ethical approval number: F/SPMC/IERB/2158). Additionally, all the study participants gave their written informed consent to be recruited in the study.
Inclusion criteria
Individuals aged 12 years or above with 2 or more warts were included in our study. Only patients with either previously untreated warts, or those who had not received treatment in the last 1 month were included.
Exclusion criteria
Immunocompromised and systemically ill patients, pregnant or lactating women, and subjects with a history of any allergy or hypersensitivity reaction to any of the components of BCG, PPD or Mw in the past were excluded from the trial.
Treatment protocol
The study participants were administered either of the 2 regimens depending upon their group. Additionally, the patients were advised against taking other alternative treatments during the study period until the end of the last follow-up visit.
Individuals in group A were given with 0.1–0.2 ml of intralesional Mw vaccine (IMMUVAC 0.6 ml - Inj., Cadila Pharmaceuticals Limited) using a 26 G needle into the base of the largest wart. Correspondingly, in group B, 0.1–0.2 mL PPD Tuberculin (TUBERSOL® S1ml inj. Sanofi, containing Five (5) tuberculin units per test dose of 0.1 mL) was injected into the largest mother wart. The therapy was repeated every four weeks either until there was complete clearance in all the warts, or until 12 weeks (total 4 sessions), whichever happened first.
Follow-up and Treatment Response
After the last session (at week 12), monthly follow-up was done for the next 3 months. The treatment response was labeled as follows: complete clearance (CC) with 100% clearance of all the warty tissue and reappearance of normal skin markings, moderate clearance (MC) with 99%–25% reduction in size or number of warts, and no clearance (NC), with < 25% reduction in size/number. Immediate or delayed adverse events were also noted at each visit. An additionally supplementary assessment to note any signs of recurrence and delayed response was also done at 6 months after the last dose.
The primary endpoint was CC of all warts at week 8 and week 12, which also included the warts that might have developed appeared or developed recurrence during the treatment period. Secondary endpoints included CC at week 24, recurrence of warts at week 12 in the patients who had clearance at week 4, and recurrence of warts at week 24 in the patients who had clearance at week 12, and the side effect profile.
The analysis of both primary and secondary endpoints was done according to an intention-to-treat (ITT) model. Randomized patients who had received at least one treatment session and returned back for at last 1 follow-up visit were included. In cases any therapy-session/follow-up visit was missed, the analysis took the status of the last-observation-carried-forward (LOCF) in order to estimate any subsequent observation points. This ITT model presumed that if a patient with warts CC was lost to follow-up, then he did not develop any new warts or experience any recurrence. Similarly, those patients who had partial or no clearance at the last visit, did not experience clearance in warts once lost to follow-up.
Dermatology Life Quality Index (DLQI) questionnaire
Along with the clinical response, the treatment outcome was also measured by comparing the change in the score of the hindi validated version of the DLQI questionnaire at first session (week 0), and at the end of the last follow-up visit (week 24). The questionnaire had 10 questions, each having a maximum score of 3 (total score 30). Every question had the following possible scores: not at all or not relevant or unanswered-0; a little − 1; a lot − 2; very much − 3. Total score interpretation was done as follows: 0–1 = no effect at all on patient’s life, 2–5 = small effect, 6–10 = moderate effect, 11–20 = very large effect, and 21–30 = exceedingly large effect. The DLQI questionnaire was supplied to all the participants at week 0 and week 24 and the improvement in QOL was noted by comparing the scores.
These questions (Q) were further divided into 6 domains in accordance with the framework of Salah, ie symptoms and feelings accompanying the disease (Q1–Q2), impairment of daily activities (Q3–Q4), effects on leisure time (Q5–Q6), effect on work/school (Q7), interpersonal relations (Q8–Q9), and impact of treatment on QOL (Q10) [5].
Statistical Tools
Statistical analysis was done using the Statistical Package for the Social Sciences version 20. Paired and unpaired t-tests, Chi-square test and Z scores were calculate wherever necessary. Categorical and continuous variables were presented as absolute numbers, percentages, mean, and standard deviation (SD). A p value of ≤0.05 was considered to be statistically significant.
Results
This intention to treat analysis included 120 subjects randomized into 2 groups with 60 patients per group. The demographic and clinical data of both groups were comparable. While 45 patients were treatment naïve, 75 had received 1 or more treatments in the past with treatment failure or relapse. The mean duration of warts in both groups was comparable (Table 1). The study was completed by 102 patients (55 in group A and 47 in group B) (Figure 1). The overall number of patients with CC in all warts (according to ITT) was 42 and 31, in group A and B respectively during the final assessment done on at week 24 (figure 3,4,5,6) (Table 2). Between the 2 groups, the difference in the clearance rate of all warts at week at 1st, 2nd, 3rd and 4th dose was not statistically significant (Figure 2). Similarly, while comparing the response in only injected warts, the rate of CC was 85.45% (N = 47) and 76.59% ( N = 36), respectively. The mean number of injections required for CC in group A were 2.38, while in group B this value was 2.56. None of the patients experienced recurrence during the ensuing follow-up period.
Table 1.
Baseline demographic data of patients
| Group A (Mw vaccine) N=55 |
Group B (PPD tuberculin) N=47 |
P value | |
|---|---|---|---|
| Age Distribution in Years | |||
| Range | 12–55 yrs | 12–60 yrs | 0.09 |
| Mean ± SD | 23.5 ± 8.4 yrs | 26.9 ± 11.7 yrs | |
| Gender Distribution | |||
| Male | 38 | 33 | 0.91 |
| Female | 17 | 14 | |
| Male:female | 2.2:1 | 2.3:1 | |
| DURATION OF WARTS (IN MONTHS) | |||
| Mean ± SD | 7.12 ± 4.01 | 8.71 ± 5.13 | 0.08 |
| Mean Number of Warts ± Sd | |||
| 12.01 ± 6.19 | 10.77 ± 7.32 | 0.35 | |
| Inference: Both the groups had comparable for baseline demographics | |||
PPD = Purified Protein Derivative of Tuberculin; Mw = Mycobacterium w vaccine; SD = standard deviation
Figure 1.
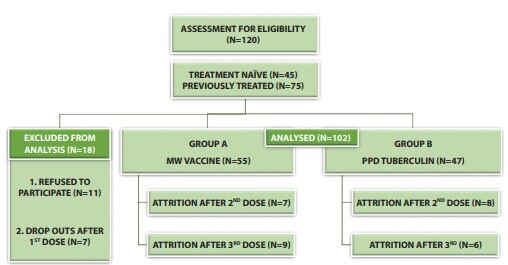
Patient selection, follow-up and attrition in an intention-to-treat analysis. PPD = Purified Protein Derivative of Tuberculin; Mw = Mycobacterium w vaccine
Figure 3.
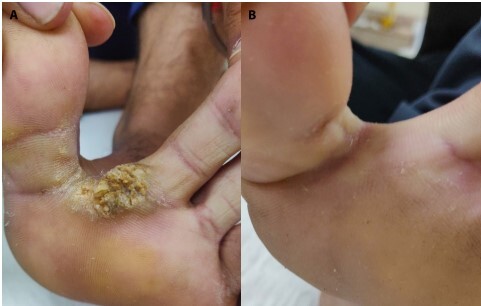
(A) Multiple interdigital warts at baseline. (B) Complete resolution after 4 doses of Mw vaccine.
Figure 4.
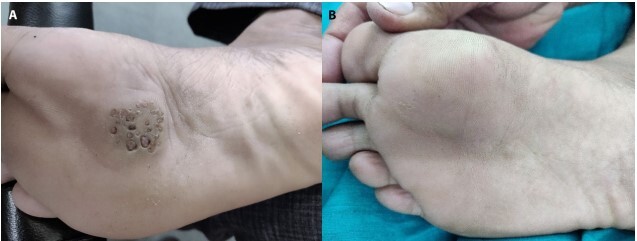
(A) Multiple myrmecia wart at baseline. (B) Complete resolution after 4 doses of Mw vaccine.
Figure 5.
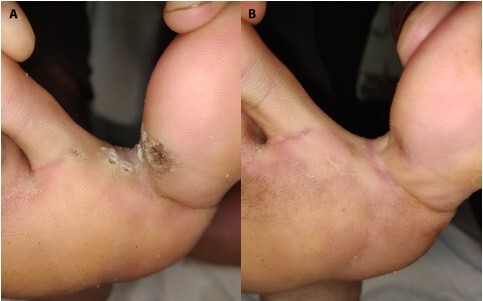
(A) Multiple interdigital warts at baseline (B) Complete resolution after 2 doses of PPD tuberculin.
Figure 6.
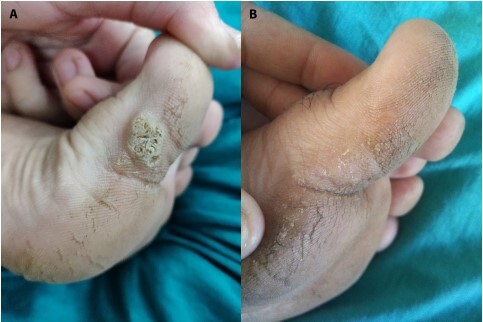
(A) Plantar warts at baseline. (B) Complete resolution after 4 doses of PPD tuberculin
Table 2.
Rate of clinical response in an intention to treat analysis
| CLEARANCE RATE IN WARTS | ||||
|---|---|---|---|---|
| GROUP A (Mw) N=55 | GROUP B (PPD) N=47 | X2 value | P value | |
| COMPLETE CLEARANCE | 42 (76.3%) | 31 (65.9%) | 5.47 | 0.064 |
| PARTIAL CLEARANCE | 9 (16.4%) | 5 (10.6%) | ||
| MINIMAL/NO CLEARANCE | 4 (7.3%) | 11 (23.4%) | ||
PPD = Purified Protein Derivative of Tuberculin; Mw = Mycobacterium w vaccine
Figure 2.
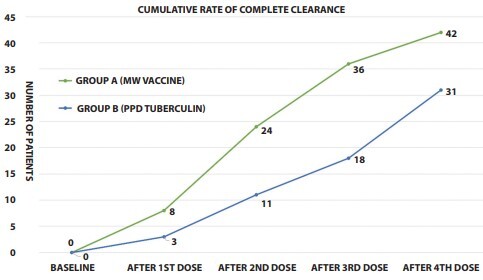
Cumulative response rate between the 2 groups.
During the analysis of the impact of other factors on treatment response, we discovered that the mean number of warts had a significant influence on the rate of clearance (P <0.01). Patients with CC from both groups combined had 9.13 mean number of warts. Meanwhile, those with moderate to no clearance (MC and NC) had 17.26 warts. There was also a significant difference in the mean duration of warts between patients who responded and those who didn’t respond to the therapy. The combined mean duration of warts in patients from both groups with CC was 7.21 months, and that of patients with MC/NC was 9.47 months (P = 0.02).
Assessment of secondary outcomes revealed a comparable adverse reactions profile in both groups. The side effect event profile of our patients was excellent with transient injection site pain and erythema being the most common adverse events. Other rare side effects included transient fever, injection site nodule formation and transient urticaria.
While analyzing the DLQI questionnaire, the most severely affected domains were symptoms and feelings accompanying the disease (Q1 and Q2), and the inconvenience experienced by patients while seeking treatment (Q10). The mean DLQI score of patients in group A and B at week 0 was 8.03 ± 1.03 SD and 7.96 ± 1.53 SD, respectively, which improved to 2.14 ± 0.77 SD and 2.71 ± 1.02 SD at week 24, respectively (P <0.01).
Discussion
The Mw vaccine is based on a cultivable non-pathogenic mycobacterium known as the mycobacterium inducus pranii, which was developed at the All India Institute of Medical Sciences in the 1970s. After more than 36 years of being tested rigorously, the vaccine was approved for the prevention of leprosy in 2019 [6]. PPD, on the other hand, is a skin antigen used for determining an immune response to tuberculosis [7]. These immunotherapeutic agents work on the principle of eliciting a Th1 mediated immune response with the production of high levels of of IL-2, IL-5, and IFN-γ.
The role of Mw for management of warts was noted for the first time by Gupta et al in genital warts with an impressive success rate of 89% [8]. Later Meena et al observed its favorable response in multiple cutaneous warts with 83% CC rates [9]. Various authors in the past have reported the effectiveness of Mw in warts to range from 55% to 93% [10–12]. While PPD was first used by Kus et al for management of warts with a success rate of only 29% [13]. Other studies have demonstrated 46% to 96% clearance rate [14–16].
We found minimal recurrence rate in our study during the ensuing 6 months follow-up period. The rate of reduction in warts was also significant and statistically comparable between the 2 groups, however, there was a higher clearance rate in patients treated with Mw vaccine. The side effect profile was also better with Mw. Serious side effects like injection site granuloma, atypical mycobacterial infection and generalized urticarial rash were seen only in PPD group. Also, the Mw group responded faster to the given treatment than PPD group.
Interestingly, there was only a marginal difference in the response rate between injected and distant warts, reinforcing our hypothesis that both Mw vaccine and PPD tuberculin could be effective even if injected intramuscularly.
One thought-provoking observation in our study was the delayed and sustained response in warts. At least 3 patients in group A and 4 in group B who only had partial clearance after the last injection, developed CC in all their warts at the end of the follow up period. It can, therefore, be concluded that in some cases both immunogens might impart a slowly developing but long-term immunity. So, the dermatologists must counsel their patients that they must wait for at least 3 months to let the immunotherapy work. It was our observation that there was a dramatic improvement in patients’ QoL following the completion of treatment. Fifty-three (96.3%) subjects in group A and 44 (93.6%) in group B were satisfied with their treatment. The most significant improvement was seen in the domain of ‘symptoms and feelings’. A majority of patients also reported a noteworthy improvement in their interpersonal relationships.
Limitations of our study included a short follow-up period, small sample size, no analysis of genital warts, absence of a control group, and no analysis of immunological parameters. Another major limitation included the fact that we couldn’t perform any HPV tests, and therefore, we couldn’t evaluate the response according to HPV subtypes. The extrapolated results of the patients lost at follow-up in our ITT model also posed a significant limitation.
Conclusion
We found PPD and Mw to be effective in the management of extragenital warts. Both immunogens have a good safety profile and lead to a significant improvement in patients’ QoL.
Informed Consent: The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published, and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Footnotes
Funding: None.
Competing interests: None.
Authorship: All authors have contributed significantly to this publication
References
- 1.Forman D, de Martel C, Lacey CJ, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30(Suppl 5):F12–F23. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 2.Sterling JC, Handfield-Jones S, Hudson PM British Association of Dermatologists. Guidelines for the management of cutaneous warts. Br J Dermatol. 2001;144(1):4–11. doi: 10.1046/j.1365-2133.2001.04066.x. [DOI] [PubMed] [Google Scholar]
- 3.Salman S, Ahmed MS, Ibrahim AM, et al. Intralesional immunotherapy for the treatment of warts: A network meta-analysis. J Am Acad Dermatol. 2019;80(4):922–930.e4. doi: 10.1016/j.jaad.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Dhakar AK, Dogra S, Vinay K, Sarangal R, Kanwar AJ, Singh MP. Intralesional Mycobacterium w Vaccine Versus Cryotherapy in Treatment of Refractory Extragenital Warts: A Randomized, Open-Label, Comparative Study. J Cutan Med Surg. 2016;20(2):123–129. doi: 10.1177/1203475415616962. [DOI] [PubMed] [Google Scholar]
- 5.Salah E. Impact of multiple extragenital warts on quality of life in immune-competent Egyptian adults: a comparative cross-sectional study. Clin Cosmet Investig Dermatol. 2018;11:289–295. doi: 10.2147/CCID.S165908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talwar GP, Gupta JC, Mustafa AS, et al. Development of a potent invigorator of immune responses endowed with both preventive and therapeutic properties. Biologics. 2017;11:55–63. doi: 10.2147/BTT.S128308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amirnia M, Khodaeiani E, Fouladi DF, Masoudnia S. Intralesional immunotherapy with tuberculin purified protein derivative (PPD) in recalcitrant wart: A randomized, placebo-controlled, double-blind clinical trial including an extra group of candidates for cryotherapy. J Dermatolog Treat. 2016;27(2):173–178. doi: 10.3109/09546634.2015.1078871. [DOI] [PubMed] [Google Scholar]
- 8.Gupta S, Malhotra AK, Verma KK, Sharma VK. Intralesional immunotherapy with killed Mycobacterium w vaccine for the treatment of ano-genital warts: an open label pilot study. J Eur Acad Dermatol Venereol. 2008;22(9):1089–1093. doi: 10.1111/j.1468-3083.2008.02719.x. [DOI] [PubMed] [Google Scholar]
- 9.Meena JK, Malhotra AK, Mathur DK, Mathur DC. Intralesional immunotherapy with Mycobacterium w vaccine in patients with multiple cutaneous warts: uncontrolled open study. JAMA Dermatol. 2013;149(2):237–239. doi: 10.1001/jamadermatol.2013.866. [DOI] [PubMed] [Google Scholar]
- 10.Chandra S, Sil A, Datta A, Pal S, Das NK. A double-blind, randomized controlled trial to compare the effectiveness and safety of purified protein derivative of tuberculin antigen with Mycobacterium w vaccine in the treatment of multiple viral warts. Indian J Dermatol Venereol Leprol. 2019;85:355–66. doi: 10.4103/ijdvl.IJDVL_549_18. [DOI] [PubMed] [Google Scholar]
- 11.Singh S, Chouhan K, Gupta S. Intralesional immunotherapy with killed Mycobacterium indicus pranii vaccine for the treatment of extensive cutaneous warts. Indian J Dermatol Venereol Leprol. 2014;80(6):509–514. doi: 10.4103/0378-6323.144145. [DOI] [PubMed] [Google Scholar]
- 12.Garg S, Baveja S. Intralesional immunotherapy for difficult to treat warts with Mycobacterium w vaccine. J Cutan Aesthet Surg. 2014;7(4):203–208. doi: 10.4103/0974-2077.150740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kus S, Ergun T, Gun D, Akin O. Intralesional tuberculin for treatment of refractory warts. J Eur Acad Dermatol Venereol. 2005;19:515–516. doi: 10.1111/j.1468-3083.2004.01176.x. [DOI] [PubMed] [Google Scholar]
- 14.Nimbalkar A, Pande S, Sharma R, Borkar M. Tuberculin purified protein derivative immunotherapy in the treatment of viral warts. Indian J Drugs Dermatol. 2016;2:19–23. doi: 10.4103/2455-3972.184103. [DOI] [Google Scholar]
- 15.Saoji V, Lade NR, Gadegone R, Bhat A. Immunotherapy using purified protein derivative in the treatment of warts: An open uncontrolled trial. Indian J Dermatol Venereol Leprol. 2016;82:42–46. doi: 10.4103/0378-6323.171650. [DOI] [PubMed] [Google Scholar]
- 16.Wananukul S, Chatproedprai S, Kittiratsacha P. Intralesional immunotherapy using tuberculin PPD in the treatment of palmoplantar and periungual warts. Asian Biomed. 2009;3:739–743. doi: 10.5372/ABM.V3I6.279. [DOI] [Google Scholar]


