Figure 2.
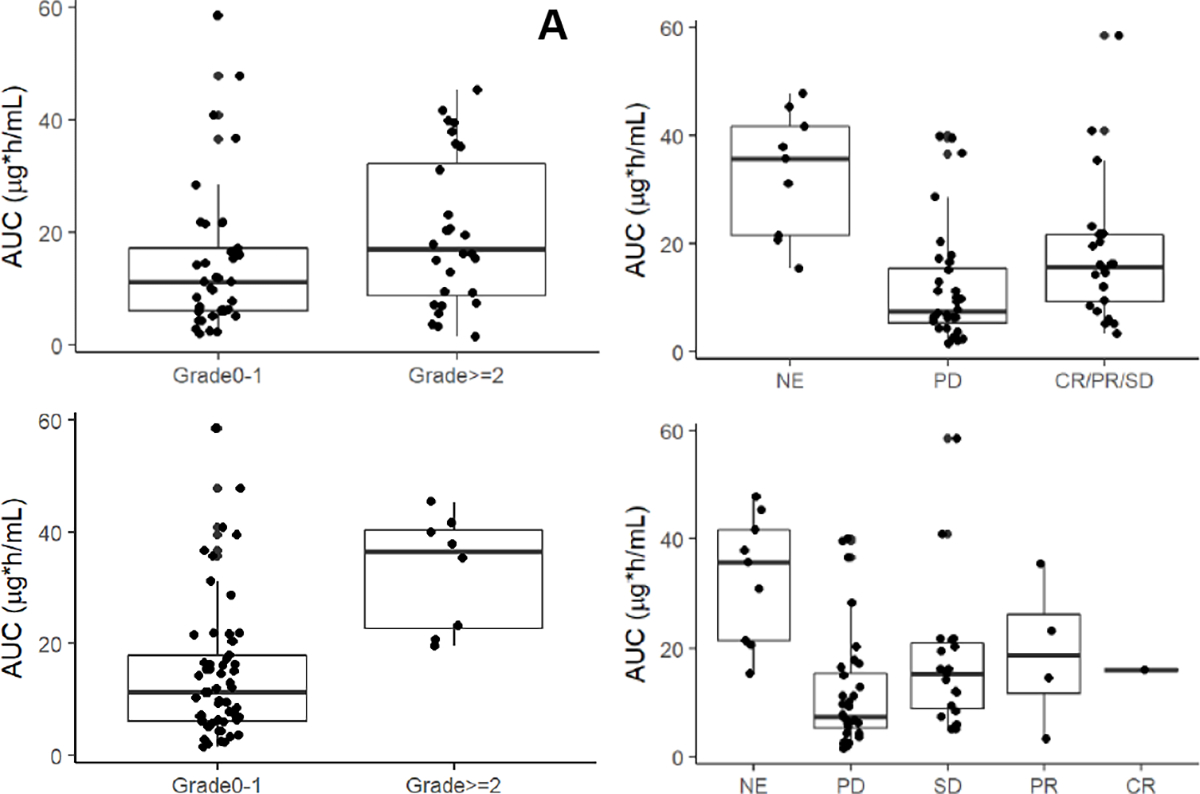
Exposure-response relationship of day 1 veliparib AUC (A) by cycle 1 any toxicity grade 0–1 vs grade 2 and up (no significance); (B) by cycle 1 nausea grade 0–1 vs grade 2 and up (p=0.0004, n=65; per Wilcoxon non-parametric test); (C) by progressive disease (PD) vs stable disease (SD), partial response (PR) and complete response (CR) (p=0.019, n=56; per Wilcoxon non-parametric test); and (D) by individual response categories (patients not evaluable for response are labelled NE).
