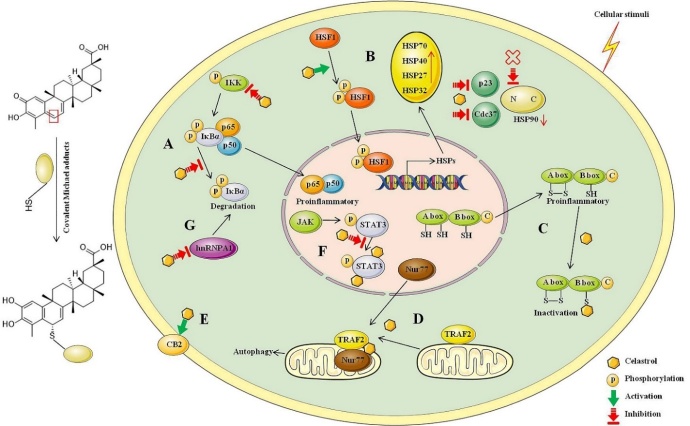
Figure 2.
Proposed schematic diagram of celastrol forming covalent Michael adducts through the binding of electrophilic sites with the nucleophilic thiol groups of cysteine residues and directly binding targets. (A) Celastrol suppresses NF-κB activation by inhibiting IKKα and IKKβ activity and inhibiting IκBα phosphorylation to decrease degradation of IκBα. (B) Celastrol activates HSF1 to up regulate a wider set of potentially neuroprotective HSPs. Different from the existing N-terminal HSP90 inhibitor, celastrol covalently binds to HSP90 co-chaperone Cdc37 and p23 to disrupt the Cdc37-HSP90 or p23-HSP90 complex. (C) Celastrol directly binds to HMGB1 and inhibits the proinflammatory activity of disulfide isoform HMGB1. (D) Celastrol promotes mitochondrial ubiquitination and autophagy by covalently binding to Nur77 and inducing Nur77 interaction with TRAF2 to inhibit the classical IKK/NF-κB pathway. (E) Celastrol is a direct and selective CB2 agonist and triggers several CB2-mediated downstream signaling pathways to reduce inflammatory responses. (F) Celastrol directly binds and inhibits STAT3 tyrosine phosphorylation and nuclear translocation. (G) Celastrol accelerates the degradation of hnRNPA1 by directly binding with it and modulates hnRNPA1-IκBα-NF-κB-TNF-α pathway.