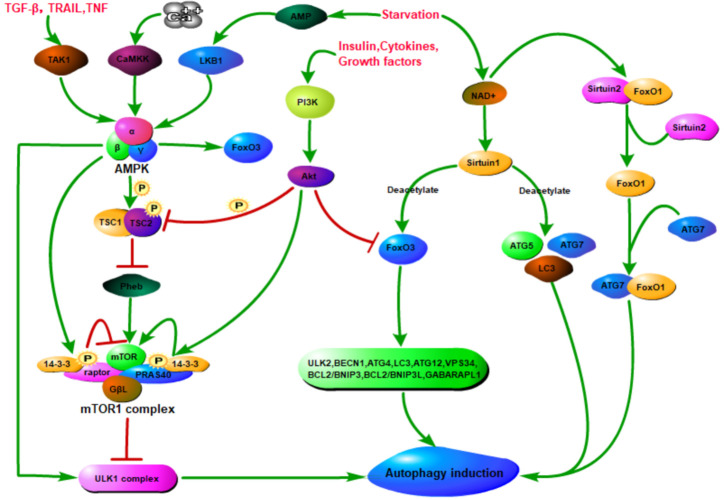
Figure 3.
Nutrient dependent autophagy regulation. Nutrition-dependent autophagy regulation is mainly related to mammalian rapamycin target complex 1 (mTORC1), adenosine monophosphate-activated protein kinase (AMPK), and oxidized nicotinamide adenine dinucleotide-dependent histone deacetylase (Sirtuin1). mTOR is the major negative regulator of autophagy. mTOR signaling pathway was activated in nutrient abundance, while AMPK signaling pathway and Sirtuin1 signaling pathway were activated in nutrient deficiency. AMPK is phosphorylated and activated by several upstream kinases, including LKB1, calcium/calmodulin-dependent protein kinase (CAMKK) and mitogen-activated protein kinase 7 (TAK1). Once activated, on the one hand, AMPK can directly phosphorylate serine/threonine protein kinase ULK1 to promote autophagy and/or directly phosphorylate raptor (a scaffold protein used to recruit mTOR substrates) to mediate the binding of 14-3-3 (a cytosolic anchor protein) to inactivate mTOR, thereby indirectly activate autophagy. On the other hand, AMPK can phosphorylate TSC2 in the TSC1/TSC2 complex, disrupt the interaction between TSC1 and TSC2, thereby inhibiting mTORC1 and indirectly activating autophagy. Rheb is located downstream of the TSC1/TSC2 complex and upstream of mTOR and acts as an exciter of mTOR and is inhibited by the TSC1/TSC2 complex. However, in the PI3K/Akt signaling pathway activated by growth factors or cytokines, Akt acts to activate mTOR by inducing the phosphorylation of PRAS40 and mediating its binding to 14-3-3. Moreover, Akt can also inactive TSC2 or inhibit FoxO3 (a transcription factor that can positively regulate autophagy) to suppress autophagy. When starvation, Sirtuin1 and Sirtuin2 are activated due to increased NAD+. Sirtuin 1 can deacetylate forkhead box protein O1 (FoxO1) to promote autophagic flux and/or directly deacetylate several essential autophagy proteins such as ATG5, ATG7 and microtubule-associated protein 1 light chain 3 (LC3) to induce autophagy. In addition, FoxO1 is acetylated after separation from Sirtuin2, and the acetylated FoxO1 promotes autophagy by enhancing its interaction with ATG7.