Fig. 2. Particle formation rates at 1.7 nm (J1.7) versus ammonia concentration at 223 K and 25% relative humidity.
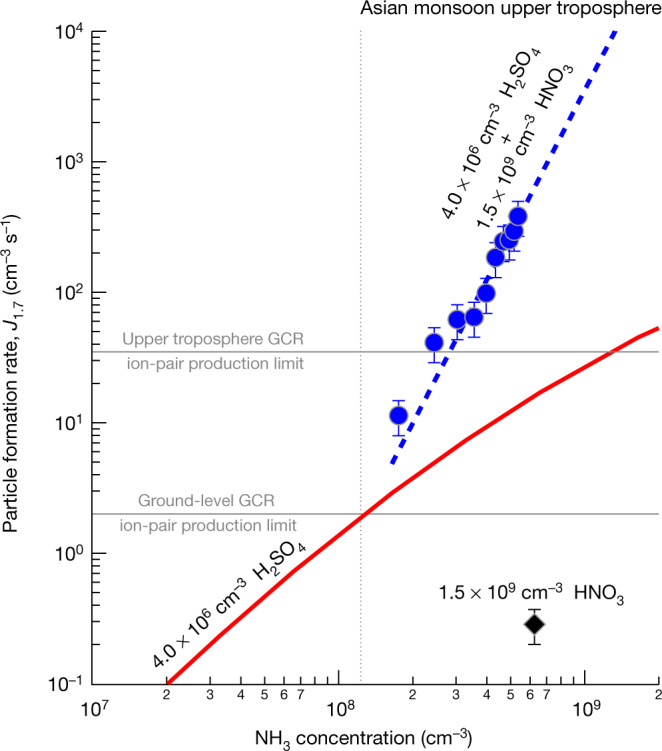
The chemical systems are HNO3–NH3 (black), H2SO4–NH3 (red) and HNO3–H2SO4–NH3 (blue). The black diamond shows the CLOUD measurement of HNO3–NH3 nucleation at 1.5 × 109 cm−3 HNO3, 6.5 × 108 cm−3 NH3 and with H2SO4 below the detection limit of 5 × 104 cm−3. The red solid curve is J1.7 versus ammonia concentration at 4.0 × 106 cm−3 sulfuric acid from a H2SO4–NH3 nucleation parameterization on the basis of previous CLOUD measurements18,19. The blue circles show the CLOUD measurements of HNO3–H2SO4–NH3 nucleation at 4.0 × 106 cm−3 H2SO4, 1.5 × 109 cm−3 HNO3 and (1.6–6.5) × 108 cm−3 NH3. The data are fitted by a power law, J1.7 = k[NH3]3.7 (blue dashed curve). The vertical grey dotted line separates ammonia concentrations measured in different regions in the upper troposphere5; the region to the right indicates the Asian monsoon conditions. The horizontal grey solid lines show J1.7 upper limits for ion-induced nucleation resulting from the GCR ionization rate of around 2 ion pairs cm−3 s−1 at ground level and 35 ion pairs cm−3 s−1 in the upper troposphere. Among the three nucleation mechanisms, H2SO4–NH3 nucleation dominates in regions with low ammonia (below around 1.0 × 108 cm−3, or 12 pptv), whereas HNO3–H2SO4–NH3 nucleation dominates at higher ammonia levels characteristic of the Asian monsoon upper troposphere. The bars indicate 30% estimated total error on the particle formation rates. The overall systematic scale uncertainties are −33%/+50% for sulfuric acid and ±25% for nitric acid concentrations.
