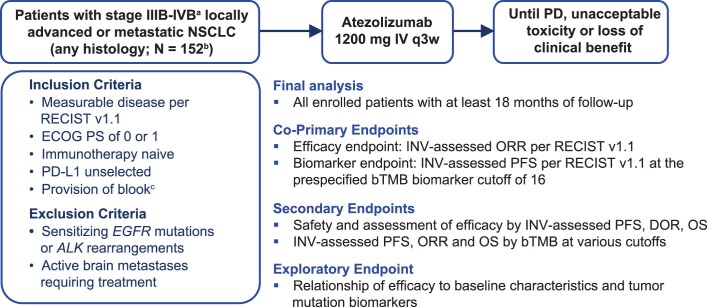
Extended Data Fig. 1. B-F1RST study design.
B-F1RST prospectively evaluated atezolizumab monotherapy in the first-line treatment of patients with NSCLC. bTMB, blood-based tumor mutational burden; DOR, duration of response; ECOG PS, Eastern Cooperative Oncology Group performance status; INV, investigator; NSCLC, non-small cell lung cancer; OS, overall survival; PD, progressive disease; PD-L1, programmed death-ligand 1; PFS, progression-free survival; RECIST v1.1, Response Evaluation Criteria in Solid Tumors version 1.1. a Staging based on International Association for the Study of Lung Cancer Lung Cancer Staging Project 8th Edition of the TNM Classification for Lung Cancer28. b Total enrolled, N = 153; however, 1 patient was never treated and was not included in the intention-to-treat population. c Tissue biopsy was optional.