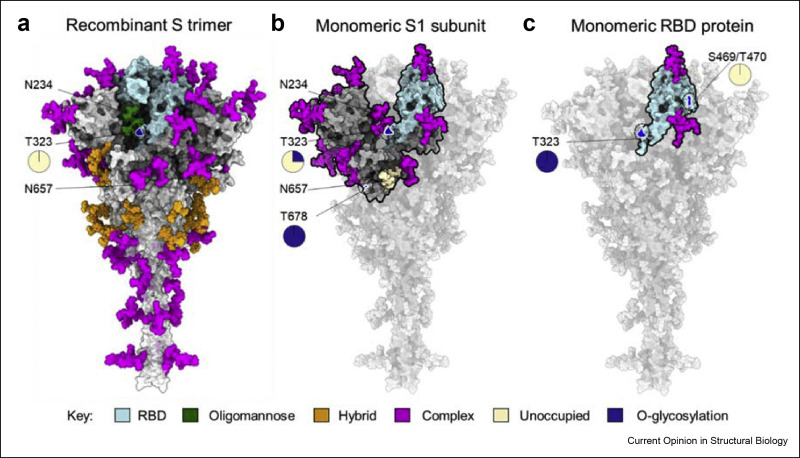
Figure 3.
The influence of protein structure on glycan maturation. (a) Illustration of glycan composition of recombinant trimeric S protein of which values reproduced from Eldrid et al. [7]. The N-linked glycosylation takes place at specific sequon, Asn-X-Ser/Thr (X is any amino acid except proline) whereas O-linked glycosylation is not dictated by specific sequon and occurs on serine and threonine in exposed regions. The N-linked glycosylation is presented in three categories on basis of oligomannose content as described in Figure 1, oligomannose (green), hybrid (orange) and complex-type (magenta) glycans. The O-linked glycosylation at T323 site (see magnification) on trimeric S is present at low levels (0.2%) of which values obtained from Eldrid et al. [7]. (b) The glycan composition of recombinant monomeric S1 subunit of which values reproduced from Wang et al. [27] and Brun et al. [39]. Most of the N-glycan sites on S1 subunit is highly processed represented in magenta except N657 which is unoccupied, represented in wheat color. The O-glycosylation is present on S1 subunit at sites, T323 and T678 (see magnification). (e) The glycan composition of monomeric RBD protein (cyan) which binds to main host receptor (ACE2). The N-glycan sites of RBD are highly processed and are represented in magenta, values reproduced from Allen et al. [38]. The O-glycosylation was observed on monomeric RBD protein at sites T323 and S469/T470 (see magnification) [7].