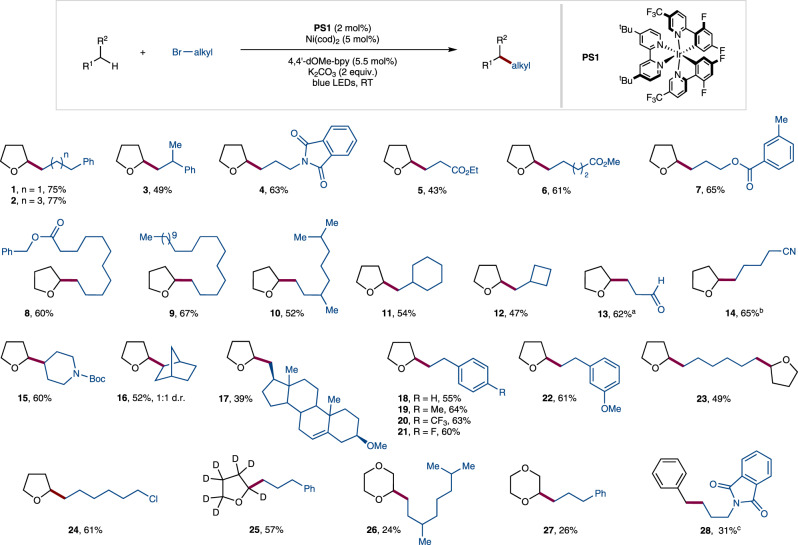
Fig. 3. Substrate scope.
Reaction conditions: alkyl bromide (0.2 mmol), C–H coupling partner as solvent (0.05 M, 4 mL), Ir[dF(CF3)ppy]2(dtbbpy)PF6 (PS1, 2 mol%), Ni(cod)2 (5 mol%), 4,4’-dOMe-bpy (5.5 mol%), K2CO3 (2 equiv.), 34 W blue LEDs, Ar, 48 h, room temperature. aThe reaction was performed using 2-(2-bromoethyl)-1,3-dioxolane and the final aldehyde product was obtained upon deprotection. bIsolated in a 5:1 ratio with the homo-coupling product of alkyl halide. c10 equiv. of alkyl component, benzene (0.125 M), NiCl2 glyme (10 mol%), 4,4’-dtbbpy (11 mol%), NaHCO3 (2 equiv.), 34 W x 2 blue LEDs, 96 h. See Supplementary Methods 3 for more details.