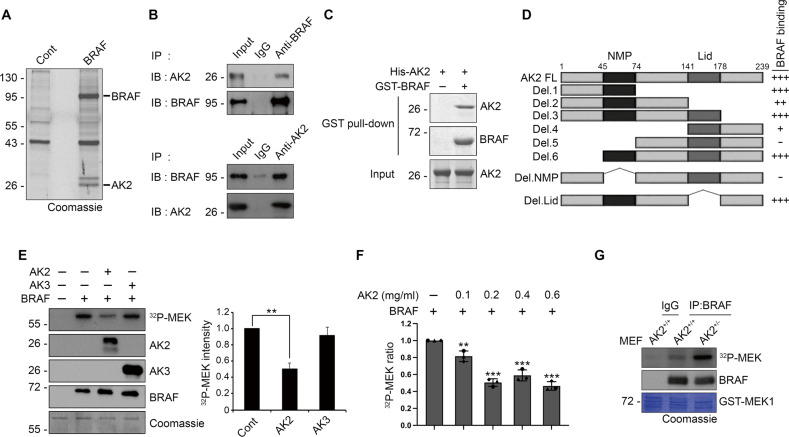
Fig. 1. AK2 interacts with BRAF, restraining the BRAF kinase activity.
A HEK293T cells were transfected with empty (Cont) or 3xFlag-BRAF (BRAF) for 24 h and cell extracts were incubated with anti-Flag antibody-conjugated to agarose bead in immunoprecipitation (IP) lysis buffer for 12 h. The immunoprecipitated proteins were then resolved by SDS-PAGE (Coomasie blue) and the bands of interests were sliced into pieces for LC-MS/MS. B Cellular interaction between BRAF and AK2. Hep3B cell extracts were subjected to immunoprecipitation (IP) assay with anti-BRAF (upper) or anti-AK2 (lower) antibody. C AK2 binds to BRAF in vitro. Purified GST-BRAF and His-AK2 proteins (each 1 μg ml−1) were combined together for 1 h and then subjected to GST pull-down assays. The pulled samples and input were analyzed by western blotting. D The region encompassing a.a. 45–74 of AK2 is essential for AK2-BRAF association. A schematic diagram of AK2 deletion mutants and a summary of their binding to BRAF are described. The blots in Fig S1E, F were quantified their signals and the values are represented; +++ (> 0.8), ++ (0.5–0.8), + (0.5–0.2), − (<0.2). E AK2 inhibits BRAF activity in vitro. GST-BRAF protein (10 nM) was incubated with either purified His-AK2 (1 μM) or His-AK3 (1 μM) protein. The kinase reaction using GST-MEK1 (1 μM) as a substrate was progressed for 2 h in the presence of 10 μM Ci [γ-32p] ATP. MEK phosphorylation was visualized by autoradiography (left upper) and the signals of [γ-32p] were quantified by densitometric analysis (right). Proteins utilized in the assay were confirmed by western blotting and Coomassie staining (left). Values are presented as mean ± SD (n = 3; **p < 0.01). F Dose-dependent inhibition of BRAF activity by AK2. BRAF protein (10 nM) was incubated with the indicated concentrations of AK2 proteins. The kinase reaction was performed as described in E. Graph bars, error bars, and dots respectively represent mean ± SD and the individual values of 3 independent experiments. Significance was determined by one-way ANOVA with dunnett’s multiple comparisons test (*p < 0.05, **p < 0.01, ***p < 0.001). G BRAF proteins were isolated by immunoprecipitation (IP) assay using anti-BRAF antibody from wild-type (AK2+/+) or AK2 knockout (AK2+/−) MEF cells and assayed for the kinase activity with γ-32p-MEK1.