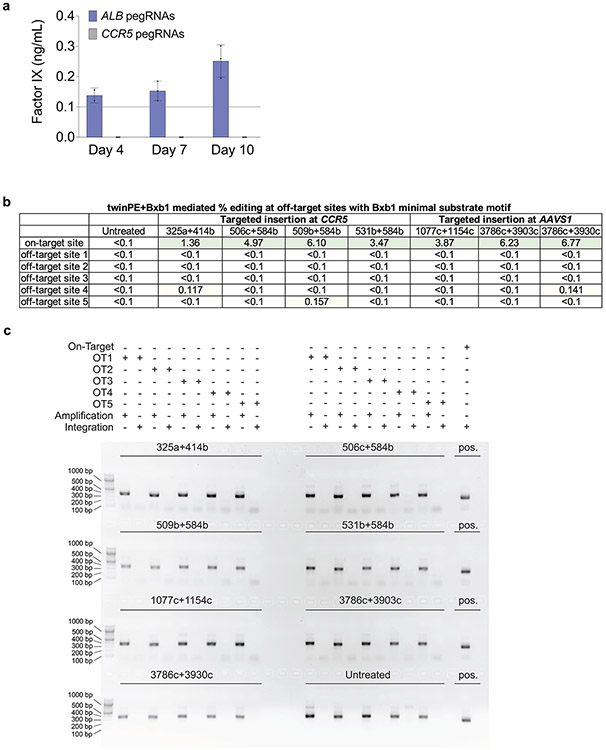
Extended Data Fig. 7. Expression of human Factor IX from the ALB promoter following twinPE-recombinase knock-in and characterization of Bxb1 off-target editing.
(a) Huh7 cells were transfected with Bxb1, donor (attP-splice acceptor-cDNA of F9 exons 2-8), PE2, and pegRNAs for installation of attB in the first intron of ALB or at CCR5. Three days post-transfection, cells were split and allowed to grow to confluence. Their media was changed, and they were left to condition the fresh media, with aliquots taken at days 4, 7, and 10. Factor IX was present at detectable levels by ELISA (dashed line represents the lower limit of detection) in two of three samples treated with ALB pegRNAs at Day 4, and in all samples treated with ALB pegRNAs at Day 7 and Day 10. Factor IX was never detected in the conditioned media of any samples treated with CCR5 pegRNAs. Values and error bars reflect the mean and s.d. of two or three independent biological replicates. (b) Targeted amplicon sequencing was performed for each of the five nominated pseudo-sites (OT1-OT5) from seven different samples treated with 5.6-kb donor DNA plasmid, twinPE reagents targeting CCR5 or AAVS1, and Bxb1 recombinase. The indels in all five pseudo-sites are either below the limit of detection (<0.1%) or near-background compared to untreated controls. The integration efficiency at the on-target site was measured by ddPCR as shown in Fig. 3d (c) To capture potential donor plasmid integration events at nominated pseudo-sites, primers were used to amplify predicted integration junctions. The gel depicts PCR reactions performed for each off-target site as indicated in the above legend. Confirmation of on-target donor integration from the samples is shown in the right-most column of the gel. In (b) and (c), two or three independent biological replicates were performed.